The recent era of celiac disease (CD) diagnosis and treatment has heralded numerous advances. Patients suffering from celiac disease now have improved chances of timely detection due to increased physician awareness and a better understanding of the diagnostic tools available, including serological and histological analysis. Furthermore, the treatment regimen of lifelong gluten free diet (GFD) has seemingly become less burdensome, if not tastier, due to a substantial increase in the number of food options prepared without gluten.
Nonetheless, post-diagnostic follow-up and adherence to GFD are critical to ensuring amelioration of all symptoms. Patients who present with persistent symptoms, especially in light of voluntary GFD adherence, remain at risk for more severe sequelae including osteoporosis, depression, anemia, and infertility. The causes of persisting symptoms can include co-morbidities such as lactose intolerance, hypersensitivity to trace amounts of gluten, intentional or unintentional diet infringement, and refractory celiac disease. This can occur quite frequently, as 2% to 10% of CD patients are nonresponders to GFD and up to 26.5% of patients report dietary transgressions.1 Inadequate or partial response to GFD can be a troubling scenario for physician and patient alike, often resulting in an increased reliance on test results to aid in the differential diagnosis. The CD test menu offered by the laboratory as well as the methodology used may impact a clinician’s ability to fully leverage non-invasive follow-up and monitoring through serological testing.
Current recommendations for diagnosis are rightly focused on sensitivity for biopsy-proven CD. Endoscopic biopsy remains the gold standard for diagnosis and is effectively a requirement for definitive diagnosis and assessment of villous atrophy in the form of a Marsh score. Patients at risk of complications from biopsy, including young children, are often candidates to avoid biopsy, especially in the presence of genetic predisposition markers such as HLA DQ2 /DQ8 and high-risk serological results as recently outlined by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHN) such as multiple antibody markers or high antibody levels.2 Serological markers which are widely used today include the following.
Antigen-specific solid phase assays:
- Anti-human tissue transglutaminase IgA antibodies (IgA h-tTG)
- Anti-human tissue transglutaminase IgG antibodies (IgG h-tTG)
- Anti-deamidated gliadin peptide IgA (IgA DGP)
- Anti-deamidated gliadin peptide IgG (IgG DGP)
Tissue-based assay:
- Indirect immunofluorescence assay using primate esophagus tissue (IgA and IgG EMA)
To one degree or another, these same diagnostic assays are often used to help guide clinical decision making in the follow-up of CD patients. A survey of Canadian gastroenterologists reported that 76% of respondents perform long-term follow-up and 65% of them include serological laboratory testing in their follow-up exams.3 Given that antibody levels typically decrease over time with the removal of gluten from the diet, levels of anti-tTG or anti-DGP antibodies will often be followed to ensure that they decline or turn negative altogether. For the purpose of monitoring, anti-DGP antibodies have been shown to be more sensitive in unveiling compliance to GFD compared to anti-tTG antibodies, even though antibodies to tTG have enhanced sensitivity for diagnosis.4 For monitoring, it becomes very important to have assays with large analytical measuring ranges and high resolution, which are precise and reproducible so that results from different time points can be compared in a meaningful manner. In the face of symptoms that are persistent to any degree, the value of assessing what, if any, villous atrophy is remaining can be critical, and it is ideally assessed without additional invasive biopsy to the very region under repair.
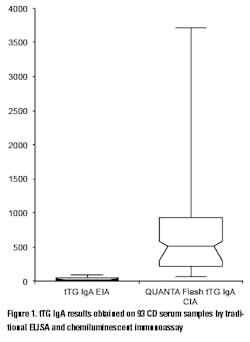
The practice of CD monitoring may be aided by advances in the methodology used for the detection of routine serological markers. The use of chemiluminescent systems has ushered in a new era of convenience, reproducibility, and sensitivity that traditional methods don’t allow. In a recent study conducted on 93 serum samples from CD patients, the tTG IgA results of 19 samples were above the analytical measuring range (AMR) with a traditional ELISA assay (thereby preventing exact quantitation without further dilutions and repeated testing), while only two results were above the AMR of a chemiluminescent immunoassay (CIA) (unpublished observation). Moreover, the range of the results of those samples that were within the AMR of the ELISA was 2.7-96.1 units, while the same samples produced 56.3-3,496 CU (chemiluminescent units) on the chemiluminescent system demonstrating significant difference in the resolution of the results (Figure 1). This high resolution coupled with high precision improves the discrimination and differentiation of even small changes in the antibody levels.
The use of chemiluminescence systems can have a significant impact on the diagnostic as well as monitoring utility for a given marker. “We have seen results using chemiluminescence that more reliably agree with biopsy results” says Maria Grazia Alessio, MD, Clinical Laboratory Riuniti Hospital, Bergamo, Italy. Dr. Alessio continues: “The rapid and sensitive decline in DGP results when gluten is removed from the diet make this new technology interesting for monitoring purposes.”
Another marker which has shown to correlate well to villous atrophy is IgA F-actin antibodies (AAA). Actin is known as the major component of microfilaments of cell cytoskeleton and it is regarded as the target of smooth muscle antibodies with the tubular pattern (SMA-T).5 Several studies have shown that the presence of AAA in untreated patients with celiac disease correlates with mild to severe villous atrophy.5,6 In addition, AAA can be a useful tool for monitoring, as levels decrease with gluten withdrawal and mucosal healing. 6 Table 1 is adapted from Bazzigaluppi, et al.7
IgA anti-actin ELISA tests are commercially available and provide an important non-invasive measurement which can be very useful to support the diagnosis of CD in cases of suboptimal histology or in cases where parents refuse consent for biopsy. Elevated levels of AAA have been found in 69% of sera from celiac disease patients with mild intestinal histology and 85.3% of sera from celiac patients with severe lesions.6 The IgA anti-actin is a potentially valuable marker often overlooked in the arsenal of useful markers for non-invasive post-diagnostic follow-up for monitoring villous atrophy and compliance to gluten-free diet.
Post-diagnosis management of celiac disease patients is a very important, and arguably underestimated, component of helping to ensure improved quality of life for those with CD. In the face of prescribed gluten-free diet a patient suffering persistent symptoms faces a stressful conundrum of possibilities including inadvertent gluten exposure, incorrect diagnosis, refractory celiac disease, possible co-morbidities, unknown degree of intestinal damage, and so on. It is at this juncture that the laboratory can play a critical role in collaborating with clinicians to provide meaningful markers and assay methodologies for monitoring. Traditional serological markers such as tTG and DGP antibodies measured by chemiluminescent methods may offer improved analytical sensitivity and reproducibility for follow-up testing to assess adherence to GFD. Other markers, like IgA F-actin, although currently underutilized, can also contribute to assessing the extent of villous atrophy. As awareness, detection, and treatment for celiac disease all improve, the laboratory’s role in facilitating follow-up and monitoring is sure to increase as well.
Gabriella Lakos, MD, PhD, is Director of Research at INOVA Diagnostics, Inc. Dr. Lakos earned her doctorate in Clinical Immunology at the University of Debrecen, Hungary, and worked as laboratory director before moving to the medical device industry.
References
- Errichiello S, Esposito O, Di Mase R, et al. Celiac disease: predictors of compliance with a gluten-free diet in adolescents and young adults. J Pediatr Gastroenterol Nutr. 2010;50:54-60.
- Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160.
- Silvester JA, Rashid M. Long-term management of patients with celiac disease: Current practices of gastroenterologists in Canada. Can J Gastroenterol. 2010;24(8):499-509.
- Monzani A, Rapa A, Fonio P, Tognato E, Panigati L, Oderda G. Use of deamidated gliadin peptide antibodies to monitor diet compliance in childhood celiac disease. J Pediatr Gastroenterol Nutr. 2011;53(1):55-60.
- Granito A, Muratori P, Cassani F, et al. Anti-actin IgA antibodies in severe coeliac disease. Clin Exp Immunol. 2004;137(2):386-392.
- Carroccio A, Brusca I, Iacono G, et al. IgA anti-actin antibodies ELISA in coeliac disease: a multicentre study. Dig Liver Dis. 2007;39(9):818-823.
- Bazzigaluppi E, Parma B, Tronconi GM, et al. IgA anti-actin antibodies in children with celiac disease: comparison of immunofluorescence with Elisa assay in predicting severe intestinal damage. Ital J Pediatr. 2010;36:25.