Improving existing QC practices
Quality control (QC) management is one of the most important tasks that takes place in the clinical laboratory. There are different regulations available for guidance, such as CLIA and ISO 15189, which outline mandatory QC practices for in the lab. Accreditation bodies – such as COLA, CAP and TJC review – audit laboratory QC processes and practices and are sources of information, too.
While QC practices should be reviewed periodically to improve or enhance them, labs usually don’t do this for well-established processes. This may be because revising a process will frequently lead to follow-up tasks and updates to documentation. If the changes are made to a practical process, changes to standard operating procedures (SOP) and retraining of the staff may also be needed.
However, new and updated guidance for improved QC practices continues to be developed and promoted. Laboratories should be aware that some of their current practices may have been updated, or their current practices in use might need revisions. This article highlights a few QC practices that themselves may be in place at some level, but might not be current with updated guidance – which can provide useful insights and other benefits, such as time and cost savings.
QC targets
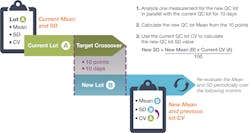
One of the most important tasks in quality control is establishing a QC target and range, or standard deviation (SD), for controls. Both the mean and the SD must be estimated by the lab when starting with a new quality control lot. The outdated historical practice of measuring 20 results over 20 days was revised in 2016 via Clinical and Laboratory Standards Institute (CLSI) C24 A4.1
Under the revised standard, a crossover study – based on a rigorous statistical analysis – can be performed faster with fewer QC materials to provide initial QC targets. By updating their practice in this regard, laboratories can also benefit from significant time and cost savings, while improving their initial QC target estimation.
Calculating the new target mean can now be done by measuring 10 QC results over 10 different days. When multiple instruments or instrument modules are running the same tests, the 10 new lot measurements are spread over the different instruments (for example: 5 QC results on two different instruments over 5 days), thus lowering the time to establish a new mean even more. There is no longer a need to establish a new SD or coefficient of variation (CV). The lab can simply use its current QC long-term CV – and use that CV to calculate the new SD. Alternatively, the lab can copy the current CV to the new lot. After a period of time, the lab would then review QC data and may update the range as indicated by performance.QC Frequency and Testing Volume
Another QC practice that has been defined by regulatory bodies is the frequency of running quality control.
Testing two levels of QC once per day has been the fixed minimum for many U.S. laboratories – with the exception of blood gas, hematology and coagulation QC, which are run more frequently. With almost all of today’s modern instruments running in a continuous testing mode, the QC schedule (or when to test QC) is now as important as how many QC processes are tested and what QC rules are applied.
To limit reporting of erroneous patient results, it is not the time between QC events that is most important, but the number of patients samples run between these QC events. If they only run their QC once per day, a lab running 50 patient samples a day and a lab running 5,000 patient samples a day have distinctly different levels of risk of reporting incorrect patient results.
It is very important to know the number of patient samples run between QC events. Based on this number, labs should attempt to make the time between QC events shorter than the time needed to recover from an out-of-control condition.2 Many labs are forced to spend extra time identifying, pulling, and retesting suspect patient results, because they allowed too much time to pass between QC procedures. The solution is to shorten the time between QC events.
QC Frequency and Critical Control Points
Besides the number of patient samples run between QC events, another important consideration in terms of enhancing QC practices is the concept of critical control points.1 For example, it is important to end a testing run that occurs before a critical event with QC. These critical events are tasks that might adversely impact the measurement procedure, such as replacing reagents, performing maintenance, or calibrating instruments. The QC process confirms that the instrument was still performing within normal specifications throughout the testing run that occurred before the critical event. After execution of the critical event, a lab should start the new run with another QC procedure to ensure the critical event did not negatively impact instrument performance.
QC procedures also should be executed at the end of the day. Many smaller laboratories will start the day with a QC run, after they have performed maintenance, replaced reagents and performed calibrations. If this process is only repeated once a day, it can become challenging for the laboratory to effectively confirm if the patient samples tested throughout the previous day were produced while the instrument was still performing within acceptable specifications. The only way to confirm this is for labs to end the day with a QC run. If this run is accepted, patient results can be reported. This process enhancement is also called “bracketing QC.”
All these suggested practices are part of the laboratory’s QC strategy, and they should be based on quality requirements and performance goals based on the risk of harm to patients. Patient risk-based QC practice guidelines, such as CLSI EP-23,4 provide a formal approach, which the laboratory can use to establish policies and procedures to help prevent or reduce patients’ risk of harm. Labs should evaluate the different kinds of malfunctions or errors that an instrument might produce and the frequency they could occur. The probability of occurrence of patient harm depends on a measurement procedure’s reliability, the effectiveness of the laboratory’s QC strategy (to limit the number of erroneous patient results that get reported when out-of-control conditions occur) and the likelihood that erroneously reported results could lead to patient harm.
With these patient-risk guidelines, the laboratory should devote more QC to analytes with a higher probability that erroneous results will lead to patient harm and also to analytes, where the expected severity of harm is higher.3 This helps to maintain the focus of the lab’s QC on patient safety, as well as instrument performance.
Conclusion
The focus of quality control is no longer centered around the instrument alone. It is important to think about the final use of the test results. Not all tests are equal; therefore, the QC strategy does not need to be exactly the same for all tests. It is easy to use the same practice across all tests (same frequency, QC rules, number of QC levels, etc.). However, this approach leads to situations in which some tests might be doing “too much QC,” while other tests might not have sufficient levels of error detection, potentially leading to patient harm if not detected in time.
Defining QC practices is an ongoing task for any laboratory. With new tests, instrumentation, methodologies and updated guidance documents emerging all the time, the availability of a good QC plan and QC practices is very important.
References
- C24 A4: Statistical Quality Control for Quantitative Measurement Procedures: Principles and Definitions, 4th Edition. CLSI., Wayne, PA, 2016.
- Parvin C. QC design: it’s easier than you think. Medical Laboratory Observer. 2013. https://www.mlo-online.com/home/article/13006005/qc-design-its-easier-than-you-think. Accessed March 31, 2021.
- Parvin C. Six QC recommendations to consider today. Medical Laboratory Observer. 2017 https://www.mlo-online.com/continuing-education/article/13009039/six-qc-recommendations-to-consider-today. Accessed March 31, 2021.
- CLSI EP23-A: Laboratory Quality Control Based on Risk Management; Approved Guideline. CLSI: Wayne, PA, 2011.
About the Author

Nico Vandepoele, BSc
serves as Scientific and Professional Affairs Manager for Bio-Rad Laboratories Quality Systems Division. He works to promote an understanding of laboratory regulations and best practices as they pertain to QC and EQA/PT programs.

Curtis Parvin, PhD
is retired from Bio-Rad, where he was Manager of Advanced Statistical Research. Prior to joining Bio-Rad, Parvin was the Director of Informatics and Statistics at the faculty of Washington University School of Medicine.