When the core lab at Carolinas HealthCare System transitioned to a fully automated MRSA testing process in early 2017, a before-and-after workflow study was implemented to assess the impact on lab efficiency, quality control, and other factors. The study results indicated that the change led to significant improvements in productivity and quality through the optimization of the test process, information flow, and the physical movement of people through the lab. Other unexpected benefits emerged as well, reinforcing the importance of continuous lab process improvement in today’s highly demanding and competitive healthcare environment, and the benefits it can provide, not only to patients but to staff as well.
Toward automated MRSA testing
Carolinas HealthCare System (CHS) is a comprehensive care system with more than 900 facilities including academic medical centers, community hospitals, and nursing homes, most of which are within a 75-mile radius of Carolinas Medical Center in Charlotte, North Carolina, although some extend as far as Charleston, South Carolina. Carolinas HealthCare System Core Laboratory, the hub of the Carolinas Laboratory Network, provides most of the diagnostic testing for the system and nearly all methicillin-resistant Staphylococcus aureus (MRSA) testing for CHS, including 13 acute care hospitals in the area. The annual MRSA testing volume at the core lab was about 72,000 in 2016, or about 200 tests per day—a five percent increase from the year before.
When CHS began its MRSA surveillance program about 12 years ago, testing was originally performed using bacterial culture. Later, the core lab transitioned MRSA testing to a polymerase chain reaction (PCR) method, while it continues to use culture for outpatient orthopedic surgery. In early 2017, lab management decided to shift MRSA testing to a fully automated PCR platform and do a before-and-after study to compare the impact on lab processes and workflow. For the fully automated process, the CHS Core lab operated a total of four systems around the clock: three shifts, 24 hours a day, seven days a week.
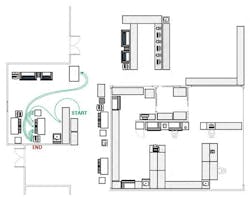
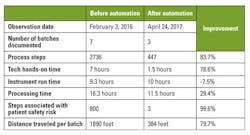
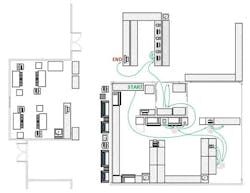
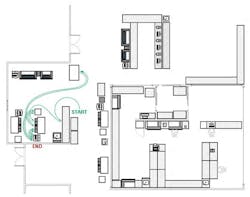
The study data summarized in Table 1 demonstrated a 79 percent reduction in technologist time. The reduction in the number of process steps (83.7 percent) was paralleled by the reduction in the distance traveled by the laboratorian per run (79.7 percent), as shown in Figures 1 and 2.
Process flow. One key change in transitioning to the fully automated platform was automating sample extraction, including heating, lysing, centrifuging, addition of master mix, and transferring the sample to the PCR carousel. Another significant change was the larger batch size. Before automation, sample preparation and testing of 200 samples a day, assuming seven batches of 30 samples each, required seven hours of hands-on time. With the fully automated system, which uses a 96-well format and therefore allows larger batches, hands-on time is 1.5 hours, an almost 80 percent reduction.
Another important finding was the reduction of risk associated with manual sample handling. The workflow study identified 400 sample prep steps (of more than 2,000 total process steps) that could be vulnerable to human error, including those that involve manual labeling or could result in misalignment of a batch order. These error-prone steps were eliminated with automation. The remaining element of risk in the automated process is in the inadvertent release of results awaiting repeats or manual review.
Physical flow. The workflow study traced the distance traversed within the lab as the laboratorians pick up patient specimens and take them through each step of the operation. By locating specimen drop-off at a centralized location in the lab, optimizing the placement of instruments and, above all, using automation, the lab was able to cut in half the distance walked by the staff member for each batch. The number of batches per day was reduced by more than 50 percent, from seven batches to three, since the automated system allows a larger batch size. As shown in Figures 1 and 2, the 2,000 feet traveled on a daily basis for the manual process has been reduced to 400 feet. With year-round operation, the reduction of 1,600 feet per day translates into more than 100 miles per year!
Information flow. Updated software enables the automated system to interface directly with the CHS laboratory information system (LIS). The streamlined resulting process, including checking controls, holding failed or invalid runs, printing results, highlighting and accepting results, and releasing to the LIS, takes a total of three minutes per run. Without the direct interface with the LIS, the process is manual and takes 20 minutes per run. Manual entry of results also introduces increased risk of human error.
The road to implementation
Our decision to fully automate MRSA testing began long before the current solution, when we chose the centralized testing model for the operational efficiencies it offers. Centralized testing also gives us the scale to make operational and economic sense for around-the-clock operation, with continuous specimen flow 24 hours a day, seven days a week, which is critical to support the patient care needs of CHS.
STAT vs. batch. In making the choice to move to fully automated MRSA surveillance testing, one of the first decisions we had to consider was STAT vs. batch testing. We had to weigh the benefits of on-demand testing, which implies potentially faster time to clinical intervention, against the potential lower costs and more streamlined workflow associated with a batch model. Studies we reviewed concluded that the turnaround-time advantage of STAT testing diminishes quickly once testing passes a volume threshold of around 10 tests a day. At the same time, reagent cost per specimen drops with an increasing number of samples in batch testing, while the one-test-at-a-time STAT cost model does not provide the same reagent cost benefit with increasing volume. Also, on-demand testing by definition requires the immediate attention of the lab staff. In the patient care setting, this means interrupting clinical workflow to respond to each test result as it comes in.
Platform migration. The migration to the new platform was not without challenges. The new automated platform uses a different patient specimen collection swab, which means a change in operation at the point of care. We designed and implemented a comprehensive communication program for the entire clinical team, in particular nurses and nursing educators. The transition to the new specimen collection swab ultimately took the full 30 days allocated in our implementation schedule.
Intangible benefits
Within two months, the transition was completed, and results of the workflow study supported our decision, which was based on improving operational efficiency and elevating quality by reducing errors. One ancillary benefit of the migration that we did not anticipate was the positive feedback from the lab staff about how automation improved their day-to-day work experience by allowing them to replace mundane, repetitive tasks with more challenging work and freeing up time for things they really wanted to do, such as being engaged in new technology. The lab staff reported a lower level of stress and greater enjoyment of their work. With a growing shortage of qualified lab personnel and the high cost of recruiting and training, this benefit cannot be overemphasized.
Looking ahead
The challenge to improve efficiencies and elevate quality will only continue with ongoing pressures on the healthcare system. Continuous process improvement is key, and workflow studies are a powerful tool for labs to accurately follow key metrics, validate decisions made, and guide future decisions.
Heather N. Todd, MLS(ASCP)CM MBCM, serves as Manager, Lab Quality, Regulatory for Carolinas Pathology Group and Carolinas HealthCare System.
John W. Longshore, PhD, FACMG, serves as Director of Molecular
Pathology for Carolinas Pathology Group and Carolinas HealthCare System.