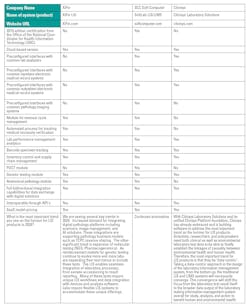
Labs are still navigating post-COVID challenges. With staffing shortages, workflow optimization demands, and artificial intelligence on the rise, choosing the right laboratory information system (LIS) is more important than ever.
The Medical Laboratory Observer LIS Buyers Guide is an annual survey that identifies LIS products and updates and changes to them. MLO asked LIS experts for their observations on the biggest changes and trends in LIS this year.
Kim Futrell, MLS(ASCP), MSHI, CPMM, Senior Strategic Marketing Manager Orchard Software, commented, “Staffing shortages, continued growth in testing menus, and a push for rapid turnaround times have forced many labs to look toward tools and equipment that can help them improve efficiency and “work smarter.” Trends that we have seen continue to gain traction within the laboratory industry (that involve the LIS) are advancements in automation, analytics, AI, and digital pathology.”
She continued, “Many labs have turned to automation tools and equipment to help speed processes and reduce errors associated with repetitive tasks. For example, we have seen a greater need for the LIS to automate insurance verification and prior authorization. Taking that further, integrated automation equipment is being used to improve efficiency, productivity, safety, and quality; and help free up laboratory professionals for less repetitive tasks.”
“We have also seen renewed interest in laboratories needing to make the most of their data, to be able to use their data to make informed business decisions. Laboratories are reaping the benefits of having aggregated data that can be analyzed for continuous quality improvement and benchmarking. We expect this to continue and the use of AI to gain traction within the industry.”
Joseph Nollar, AVP of LIS Product Management, XiFin, Inc, noted several key LIS trends in 2023. “Device integration requests to assist in streamlining lab automation are at an all-time high. For example, in the pathology market there is a significant demand for integrating with histology automation platforms. This often includes integration with digital pathology platforms including scanners, image management, and AI solutions,” he said. “These integrations are supporting new pathology business models where pathologists are partnering with physician groups and labs as independent contractors, and they want digital pathology to support their new business models. With this digital pathology expansion there is a corresponding expansion into novel artificial intelligence algorithms for diagnosing cases. For example, running FDA-approved AI algorithms on prostate stains digital imaged to give insightful information to pathologists. AI and digital pathology are providing an opportunity for labs to offer new testing capabilities and insightful data to the marketplace.”
Nollar commented on another growth area that they are observing at XiFin: Molecular Testing, specifically NGS and Pharmacogenomics. “As reimbursement models for genetic testing continue to evolve more and more, labs will be expanding their test menus to include these tests. Efficient laboratory workflows are critical to meet the growing demand for molecular testing. The LIS enables seamless integration of laboratory processes, from sample accessioning to result reporting. Many of these tests require unique LIS workflows and data integration with devices and analysis software. Labs require flexible LIS systems to accommodate these unique offerings.”
Current issues observed
Nollar said workflow is the key to success in any LIS. “The ability to configure a workflow for any given test, type of test, or business model is critical. This capability is often the difference between success and failure.”
He continued, “For XiFin workflow is the foundation to solve many emerging lab trends including:
· Digital pathology
· Technical and professional (TCPC) revenue sharing programs
· Clinical research/trials projects
· NGS and pharmacogenomics testing”
Futrell noted that Orchard Software is working on ensuring that their customers stay up to date on the latest version of LIS software. “This improves supportability and compatibility with the latest operating system. We are proactively reaching out to our customers and having that conversation about the importance of staying up to date. There are myriad benefits to being on the most recent versions such as system stability, access to new features, and modernization of infrastructure.”
Mark Winneberger, Sales & Business Development, Electronic Lab Logs said, “Two of the most significant issues in LIS, in my opinion, are lack of offering for important modules that frequently produce deficiencies in inspections, and laborious implementation that requires significant bandwidth from the laboratory and IT teams.”
Lab Logs navigates these issues by solving some of the most deficient processes in the lab, instrument maintenance tracking and documentation, he continued. “We also complete onboarding and training for all clients, to ensure these laboratories are set up as lean and efficient as possible, and not take away from the bandwidth or other resources of the lab team.”
Service and support
Lab Logs is working to fill a large gap in most LIS offerings, by helping labs upgrade from paper processes for tracking instrument maintenance, QC, and other tasks, and improve the overall standard of quality, said Winneberger. “Laboratories that use Lab Logs in conjunction with most other LIS offerings are able to eliminate almost 100% of paper, reduce non-compliance, and maintain inspection readiness. “Lab Logs offers U.S. based customer support that is included in our subscription-based offering for all clients. Our Support team includes staff with decades of clinical lab experience that help assist all users with any questions. Lab Logs is also continuously improving, and our clients get free upgrades as we release updates to improve the product and stay ahead of the latest trends in the clinical lab industry.”
Futrell added that Orchard Software offers many options for support. “Orchard’s technical support is available via telephone, online, or email 24/7/365. During business hours, approximately 98% of all opened tickets are responded to within two hours. During off hours approximately 98% are responded to within four hours. One-on-one technical assistance from our highly proficient support team is available whenever our customers need guidance. Also, with any of our support options, software upgrades are included and available as they are released.”
She continued, “In addition to our standard Silver, Gold, and Platinum support levels, we offer special project packages, a technical administration option for laboratories that have staff who are comfortable with primary system administration but would like consultation and support for more advanced areas of the system; and a system administration program for full system oversight. All customer support interactions are tracked in our online Orchard Resource Central site, a “treasure chest” of resources.”
Nollar said, “XiFin’s SaaS-based LIS allows labs to receive software updates via the cloud and lets them take advantage of innovative new features without the need for costly upgrades. XiFin LIS also integrates with different lab instrumentation and software for streamlined laboratory operations, reduced errors, and improved efficiency.”
He noted that customers get regular software updates with their subscription model. “All clients are on the same version of the software, so your LIS is never out-of-date. There are never costly fees for updating software or hardware. The XiFin support team provides support and training to all customers on-line and via the phone covering all US time zones. Emergency after-hours support is also available. Customer service also has access to XiFin subject matter experts to assist in guiding the lab through problems relating to the LIS.”
Looking to 2024
MLO asked Futrell and Nollar what they thought was on the horizon for LIS in 2024. Here is what they predicted:
Kim Futrell, MLS(ASCP), MSHI, CPMM, Senior Strategic Marketing Manager Orchard Software: “One area where we expect to see continued growth is in the laboratory’s involvement in antimicrobial stewardship. CDC’s Core Elements of Antibiotic Stewardship provides guidance to improve antibiotic use and, subsequently improve patient safety and outcomes. We anticipate regulatory changes for Antimicrobial Use and Resistance (AUR) implementation that will include metrics that need to be communicated via LIS interface. In addition, we expect to see an increase in the use of AI algorithms to predict patient outcomes.”
Joseph Nollar, AVP of LIS Product Management, XiFin, Inc: “With the exciting increased demand in digital pathology solutions there could be shortages of scanning devices as well as a backlog of installation requests for lab information systems and image management and analysis software. If digital pathology is going to be part of your lab strategy lab managers will want to get in line as early as possible. It is better to be early to the party than late.”