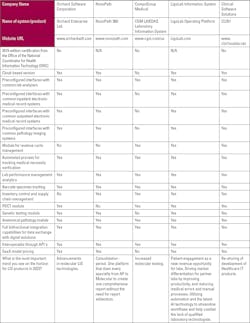
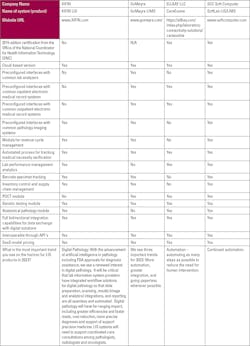
Choosing a laboratory information system (LIS) vendor is an important process that will affect a laboratory for a long time. There are several factors and issues that laboratories must consider when making the decision. Medical Laboratory Observer asked a number of LIS experts for their insights on choosing a vendor.
Bringing awareness to issues
Tony continued, “Configuration uses predefined, prevalidated process flows and graphical interfaces to create a sequence of steps for handling test samples according to the laboratory’s needs. The core system software code is not rewritten to implement these steps. Customization involves rewriting the underlying programming language to meet the lab’s workflow requirements, which can impact the system validation and regulatory compliance already in place. Many larger LIS vendors advocate only configuration — the approach embodied in their LIS. If the software provider does offer customization, the lab may wait months until the work is completed, again losing prospective customers in the process. GoMeyra resolves this issue with a dual approach: pre-configuration to handle the majority of a lab’s requirements, then customization only where necessary. This enables much faster installation and startup while meeting 100% of the client’s parameters.”
Futrell also brought to light the issue of security threats. “The healthcare industry as a whole is a target for cyberattacks and the lab handles an enormous amount of protected health information (PHI) that must be kept secure and HIPAA-compliant. To keep sensitive data secure, the LIS has to be continuously up to date on the latest threats.”
Tips for choosing a vendor
Choosing the right LIS vendor is essential. Tony, Futrell, and Avunjian all emphasized that many laboratories stick with their LIS provider for a long time, so choosing one you can trust is even more important.
Tony said, “Your LIS software provider should serve as a knowledgeable, trusted IT partner throughout the system specification, purchase, and installation process. Selecting a supportive, customer-focused LIS supplier ensures that any issues arising during system installation and startup will be quickly resolved. You can also count on them for ongoing service and support.”
Tony advises labs to select a vendor who:
- Observes your daily operations, listens carefully to your needs and concerns, and proposes a system tailored to your lab’s unique requirements and available budget
- Offers both configuration and customization of LIS functions, real-time repairs, upgrades and patches
- Makes customer service available 24 hours a day to handle performance issues or answer questions
- Responds to change requests as your operations evolve.
- Futrell advises doing considerable research and introspection before deciding whom to partner with. She also advises before reaching out, laboratories consider the following:
- Determine your budget.
- Think about what you want the LIS to do. Make a list of the functionality and features you require. Are there any “deal-breaker” or must-have requirements?
- Consider using the request for proposal (RFP) process to vet candidates.
- Research LIS vendors. Do any of the vendor candidates resonate with you and your lab’s mission?
- Ask your peers within the industry about their LIS pros and cons.
- Avunjian said, “Don’t look for a vendor but a partner, as laboratories cannot afford to switch systems often.”
- He posed some questions laboratories should ask when searching for an LIS vendor:
- Does the LIS vendor also offer a billing solution and supporting modules? If yes, does the billing solution share the same database and software infrastructure?
- Can the LIS be deployed on the cloud and on-premises, with what’s best for the lab ultimately being the determining factor for server location?
- Can the LIS manage all departments and operations with no data silos?
- Does the LIS support specimen tracking with the automatic generation of a unique barcode identifier for every specimen and document?
- Does the LIS support rules and automation that streamline workflows and reduces manual touchpoints?
- Does the LIS support fully customizable lab reports based on customer preferences and multiple report delivery options?
- Does the LIS have an interface engine, or is middleware needed for interfacing?
- Does the LIS have a comprehensive and searchable audit trail that logs and archives every activity in every department?
- Does the LIS provide insight and visibility in the forms of statistical dashboards and dynamic reports?
- Does the LIS support laboratory outreach with both client and patient portals?
- Does the LIS support all established regulatory protocols and offer compliance verification at every stage?
2023 predictions
In our annual LIS Buyers’ Guide Survey, MLO asked respondents what they saw on the horizon for LIS products in 2023. Additionally, MLO asked Tony, Avunjian, and Futrell what upcoming trends/needs they predict in LIS for 2023.
GoMeyra sees automation and integration accelerating in 2023. “Both automation and integration in LIS software architecture are becoming increasingly important for future-proofing labs,” Tony said.
Avunjian also pointed to automation trending in 2023. “With a well-documented shortage of qualified technologists and technicians to staff labs, and the cost to employ them constantly going up, more and more labs will turn to automation as a replacement for manual processes in 2023. By investing in automation, they will help relieve the staffing burden and gain more cost certainty. The LIS is the heart of lab operations, and with an all-in-one platform, redundancy is removed, workflows are streamlined, and full visibility into the operations of the laboratory is gained,” he said.
Additionally, LigoLab predicted technology advances in 2023. “By embracing direct-to-consumer patient portals coupled with telemedicine capabilities, labs will no longer be faceless. Instead, they’ll be able to directly connect with patients and satisfy their growing demand for easy and convenient access to a marketplace that offers enhanced specialized laboratory services. In addition to having easy and convenient on-demand testing, patients will also need assistance to better understand their lab reports and test results. In 2023 they’ll be able to get this in the form of remote sessions with a medical professional connected to the patient portal,” Avunjian said.
Futrell noted that, “Going forward, continued consolidation is expected as healthcare organizations and laboratories combine services to achieve the economy of scale needed in the current healthcare landscape. LIS vendors will need to be prepared to help their lab customers grow their services and integrate across locations and organizations.”
She concluded, “The LIS is essential in today’s laboratory and a strong LIS can make the jobs of laboratory professionals much easier, which improves employee satisfaction and retention. Having a vendor that you can trust and rely on is essential and can help ease the workload burden on lab and IT staff.”