The role of medical devices in the data management processes of the modern clinical laboratory
As the modern clinical laboratory becomes more connected, it becomes increasingly difficult to efficiently exchange and manage data. This is especially true with regard to interoperability, where data is exchanged among several clinical systems.1 Yet diagnostic laboratories have become such an integral part of the connected healthcare paradigm that methods for expanding their scalability, improving performance, and managing data are critical to achieving the core objectives of meeting the needs of clinicians and patients.
Laboratories have long stressed efficiency, safety, and quality in the management of diagnostic data; however, the focus has primarily been on the analytical phase.2 But the trends toward lab automation and increasing testing require that laboratories constantly adapt to ever-changing data management requirements in all phases of the testing process. This article addresses pre- and post-analytical data management processes; discusses challenges such as patient safety, complex workflows, and reporting responsibilities; and examines how data management features of analytical medical devices can have a positive impact in today’s connected clinical laboratory.
Quality data management
In recent years, the business of operating the diagnostic laboratory has changed dramatically. The lab is a connected production environment and a primary generator of data, which requires the delivery of increased efficiency driven through LEAN workflows. At the same time, the lab is expected to improve the reporting of quality diagnostic information to the clinical staff at optimal turnaround times.3 Today’s laboratory must effectively accommodate the production, management, and transmission of diagnostic information.
To meet expectations, laboratories have implemented laboratory information systems (LIS) as well as demanded analytical medical devices with more powerful and sophisticated data management solutions.4,5 These medical devices can integrate with the LIS to manage a majority of the non-value added pre- and post-analytical data management processes, from the initial ordering of tests to the reporting of test results to the clinical staff. Data management processes found in the pre- and post-analytical phases can tremendously impact the quality and efficiency of the diagnostic laboratory.2 Integrated medical devices can help coordinate these processes, from managing test orders, to monitoring samples, to validating final test results.
A new class of molecular medical devices
Increasing awareness of the role of medical devices in improving the management of diagnostic information, as well as their ability to optimize workflow and improve turnaround time, has resulted in increased focus on more sophisticated medical devices in molecular diagnostics.7 In turn, this has led to trends in molecular medical devices such as random access testing, bi-directional interfacing capabilities, and advance order and results data management features associated with LIS integration.
Traditionally, random access and bi-directional LIS interfaces are characteristic of analyzers in the core laboratory (e.g., chemistry and hematology). However, with the trend toward random access instead of batch testing in molecular diagnostics, bi-directional LIS functionality has found its way into the diagnostic lab.5,7 The relationship between bi-directional interfaces and random access testing presents several implications for diagnostic laboratories in terms of detecting errors and improving pre- and post-analytical data management processes.
Despite the data management benefits of a connected medical device, a key requirement is to accommodate standard messaging protocols, such as HL7 and ASTM, for interoperability in information exchange. From an interoperability perspective, medical devices that support bi-directional interfaces and standard messaging protocols enable more effective information flow with the LIS and other clinical information systems.8
Considering all the above‐mentioned benefits and challenges, it is evident that medical devices with the most flexible and connectivity-based features are more suited to play a greater role in meeting the data management needs of the modern diagnostic laboratory.
Pre-analytical data management
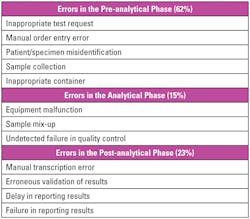
The clinical laboratory is a relatively high-risk environment with the possibility of error in several key areas of the testing process. Errors are more significant in the pre-analytical phase (Table 1), which can significantly affect patient safety and quality of care.2,6 Focusing on the pre-analytical steps that are presented in the laboratory, errors can occur when test orders are programmed into the instrument, in specimen identification, or in specimen receipt. Medical devices with bi-directional LIS interfaces can help laboratories optimize workflow and reduce errors by managing some of these pre-analytical data management steps.
A bi-directional instrument simplifies test order management and automates test order entry, which saves the laboratorian the time to program test orders into the instrument and eliminates manual entry errors. Particularly, instruments with advance broadcast bi-directional capabilities provide process controls that allow staff to load any specimen and be assured that the proper test information is available to process the specimen, which eliminates the risk of order misinterpretation or incorrect container. These capabilities are particularly important in high-throughput laboratories facing staff shortages.9
In high-throughput laboratories, medical devices with advance data management features present significant process controls and workflow optimization to minimize the potential for error in the total testing process. Consider an instrument without bi-directional capability. Following the transmission of the clinician’s test order, the laboratory requires that specimen transport, preparation, and sample accessioning happen prior to the analytic phase. All of these are manual steps that are prone to error.
For example, if a specimen is not properly accessioned, it could take multiple hours until the ordering clinician realizes the test result has not been reported within the expected turnaround time, and before the lab is even aware of having to locate a misdirected specimen. If or when the specimen is located, it must be processed, likely as a STAT result, given the delay before realizing the sample has not yet been run.
In contrast, with an instrument that has advance data management features such as bi-directional LIS, an interfaced medical device would have visibility to the queue of pending test orders from the LIS. In addition, considering an instrument that has intelligent alert notification features that the laboratory staff can configure, if a specimen was not processed by the instrument within a pre-set period of time, an alert could be sent to the relevant supervisor or laboratorian, proactively making him or her aware of the overdue pending test order and allowing him or her to take action before receiving a call from the floor. These types of advance capabilities can help reduce errors such as lost specimen and delayed reporting of results, and can help improve laboratory service levels and customer satisfaction.
Post-analytical data management
In the post-analytical phase, results are released from the instrument to the LIS. They are then released to the clinical staff based on the auto-verification policy of the laboratory. At this phase, errors in result reporting from the instrument are of concern. Some areas of potential error include transcription of results and result validation.10
Bi-directional medical devices with advance data management features can automate post-analytical steps to eliminate reporting errors and improve the turnaround time of results.8,10 These medical devices can manage results reporting by applying rules and logic to either auto-validate results for auto-release to the LIS, or hold specific or unusual results for review or rerun. With auto-validation features, the laboratory staff is not required to review every result, and the turnaround time for non-infectious patients can be improved, both helping streamline bed management and ED wait times and allowing the clinical staff to focus on patients who require intervention such as therapy or isolation. Given this level of process control, medical devices with rules-based auto-validation features can help to improve staff productivity, since the manual review of result reports is consistently performed without manual intervention, which in turn also optimizes workflow.
Considering all aspects
As diagnostic laboratories look to fine-tune their approaches to optimize data processing, staff productivity, and workflow, they should consider all aspects of the testing process to improve quality, productivity, and patient safety. The data management capabilities of medical devices and the benefits they provide when integrated with the LIS support laboratory productivity, enhance patient outcomes by streamlining results reporting, and reduce the risk of medical errors that may lead to adverse events.7 Advanced data management solutions and the benefits they can bring should be an increasingly important consideration in the evaluation and assessment of laboratory equipment.
REFERENCES
- Beckwith B, Aller R, Brassel J, Brodsky V, de Baca ME. Laboratory interoperability best practices: ten mistakes to avoid. Northfield, IL: College of American Pathologists; 2013.
- Hawkins R. Managing the pre- and post-analytical phases of the total testing process. Ann Lab Med. 2012;32(1):5-16.
- Goswami B, Singh B, Chawla R, Gupta V, Mallika V. Turnaround time (TAT) as a benchmark of laboratory performance. Ind J Clin Biochem. 2010;25(4):376-379.
- Boyd JC, Hawker CD. Automation in the clinical laboratory. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th edition. St. Louis, MO: Elsevier Saunders; 2012. pp. 478–483.
- Clark G, Anderson-Cabel L, Diggle M. Random access molecular diagnostics—Increased efficiency in laboratory workflow and translation of reduced result turnaround time to patient benefit. J Clin Virol. 2016;Volume 82 S47.
- Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem 2010;47(Pt 2):101-10.
- Blank GE, Virji MA. Development and implementation of an electronic interface for complex clinical laboratory instruments without a vendor-provided data transfer interface. J Pathol Informat. 2011;2:14.
- Venco F, Yaskin Y, Ceol A, Muller H. A LIMS for handling next-generation sequencing workflows. BMC Bioinf. 2014;15 Suppl 14:S3.
- Dauwalder O, Landrieve L, Laurent F, de Montclos M, Vandenesch F, Lina G. Does bacteriology laboratory automation reduce time to results and increase quality management? Clin Microbiol Infect. 2016;22(3):236-243.
- Sciacovelli L, Aita A, Padoan A, et al. Performance criteria and quality indicators for the post-analytical phase. Clin Chem Lab Med. 2016;54(7):1169–1176.
Shawn Hall, MS, serves as a System Solutions Specialist at GenMark Diagnostics, where he manages instrumentation and clinical systems integration. He has also worked for Montefiore Information Technology in the LIS group, where he developed core experience in diagnostic data management and instrument integration.