Today, hundreds of tests once considered too complex for point-of-care testing (POCT) are routinely performed outside the laboratory.1 Due to hospitals’ decentralized structure, laboratory testing is performed on a multitude of POCT devices from various manufacturers in many hospital wards, critical care departments, clinics, and physician offices. Typically, POC devices in a hospital can include dozens of blood gas analyzers, urine chemistry and cardiac marker systems, and handheld coagulation instruments, as well as hundreds of glucose devices.
Perceived barriers to implementing POCT have been attributed to accountability factors such as quality control, adequate staff training, and oversight for accreditation purposes.2 This article will review accreditation requirements and advances in open, vendor-neutral POCT data management to facilitate billing capture, regulatory compliance, and inspection preparedness.
Why POCT?
Because of its convenience, timeliness, and potential to improve patient outcomes, POCT’s popularity continues to rise.1 Near-patient testing increases the likelihood that healthcare professionals and the patient will receive test results faster, which may facilitate faster diagnoses, more timely treatment interventions, and improved patient compliance.
For example, a large, retrospective cross-sectional study of diabetic patients found that the availability of POCT not only lowers HbA1c in the short term (<1.5 years) but also in the longer term.3 Reduced HbA1c indicates improved glycemic control and lowers the patient’s risk of diabetic complications.3
Since diagnostic testing makes up two to three percent of healthcare costs and drives nearly 70 percent of clinical decision making, it is essential that laboratorians and POCT operators deliver quality results.4
The POCT regulatory environment
The clinical applications for POCT continue to expand, as does the identity of the staff who may conduct the testing and the regulatory requirements that apply.5 For POCT devices operating under the central laboratory license, the single biggest challenge to the adoption of POCT is maintaining control, regulatory compliance, and training records for thousands of operators performing testing on hundreds of devices in anywhere from 30 to 50 locations within the hospital system (Figure 1).6 As analysts and hospital associations predict no slowing of hospital and health system consolidation, POCT challenges are anticipated to continue.7
In the United States, all clinical testing, no matter where it is performed, is regulated by the Clinical Laboratory Improvement Amendments of 1988 (CLIA).8 POCT typically refers to CLIA waived or nonwaived laboratory tests performed at remote locations by non-laboratory personnel.5
Testing sites may choose to have CLIA inspections, or to be accredited and inspected by organizations including The Joint Commission (TJC, formerly JCAHO), College of American Pathology (CAP), or the Commission on Office Laboratory Accreditation (COLA). The professional organizations inspect laboratory members using their own standards, which the U.S. Centers for Medicare and Medicaid Services (CMS) have reviewed and found to be at least equal to CLIA standards.8 The International Standards Organization (ISO) was introduced to healthcare organizations when the CMS approved Det Norske Veritas (DNV) as a deeming authority for Medicare certification and payments. DNV was the first new deeming authority named by CMS in more than 40 years.9
ISO 15189 specifies requirements for quality and competence in medical laboratories. It can be used by medical laboratories in developing their quality management systems and assessing their own competence in the laboratory and POCT. It can also be used for confirming or recognizing the competence of medical laboratories by laboratory customers, regulating authorities, and accreditation bodies.10
While Federal regulation of POCT is minimal, states and accrediting agencies often impose additional requirements on POCT.5 Regulatory requirements for POCT generally focus on two areas: (1) training and competency of the personnel doing the testing and, (2) verification of strict adherence to the manufacturer-specified procedure for each test. The latter focus is particularly important because waived or moderately complex laboratory methods, both of which can be performed by non-laboratory personnel under certain circumstances, become highly complex if used in a manner that deviates from the FDA-approved manufacturer’s protocol. Since high complexity essentially eliminates a laboratory test from consideration for POCT, it is essential that supervision of POCT includes verification that testing procedures do not deviate from the manufacturer’s instructions.5
Managing POCT compliance
POCT supports the laboratory by delivering timely information—with the confidence of effective quality controls—to physicians at the most valuable touch points with patients. To achieve these controls, POC device manufacturers introduce connectivity systems to maximize efficiency and improve clinical outcomes through remote instrument and operator oversight.
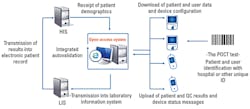
In the past, the challenge of maintaining separate POCT data management systems for each manufacturer’s products to interface with the hospital and laboratory information systems (HIS and LIS) has added complexity and increased software licensing costs. Today, hospitals can utilize an open-access data management system to connect more than 160 POC devices from all manufacturers to the hospital’s IT system (Figure 2). A manufacturer-independent solution helps ensure IT investment protection in the event a hospital changes POC equipment vendors.
While the majority of POCT done today is performed using instruments, or is migrating to instrument reading to reduce subjectivity in result interpretation and transcription errors,11 open-access data management systems are available that can support reporting of visual-read tests and facilitate billing capture.
An open-access data management system can automatically validate and transfer patient results obtained from POCT devices to the electronic medical record and monitor and manage data, POCT devices, and operators. POC coordinators can now proactively manage organization-wide EQA results according to accreditation requirements. Distributions and statistics are easily viewed and filtered with the familiar proactive traffic-light display that flags noncompliances in any connected POCT devices at any site.
The content of e-learning courses and tests, supplied by each POCT device manufacturer to the open-access data management system, guarantees that only approved content is used for training. When an operator passes the test, indicating successful completion of a course, the results are automatically documented in eLearning, and a message is sent to the data management system, which automatically extends the operator’s certification for another year.
Advances in POCT connectivity offer capabilities to address accountability factors currently perceived to be barriers to adoption. The use of connectivity can greatly improve efficiency when managing different aspects of regulatory compliance. An open-access data management system is a key enabler for POCT coordinators, by connecting devices from any manufacturer and providing operator oversight so testing efficiency is maximized, clinical workflow is improved, compliance is adhered to, and costs are efficiently managed.
REFERENCES
- Paxton A. How POC testing is pushing the envelope. CAP Today. April 2014. http://www.captodayonline.com/how-poc-testing-is-pushing-the-envelope/.
- Shaw JL. Practical Challenges Related to Point of Care Testing. Practical Laboratory Medicine. 2015;(4):22-29.
- Petersen JR, Finley JB, Okorodudu AO, Mohammad AA, Grady JJ, Bajaj M. Effect of point-of-care on the maintenance of glycemic control as measured by A1C. Diabetes Care. 2007;30(3):713-715.
- Rohr U-P, Binder C, Dieterle T, et al. The value of in vitro diagnostic testing in medical practice: A status report. Wang Y, ed. PLoS ONE. 2016;11(3):e0149856. doi:10.1371/journal.pone.0149856.
- Camacho-Ryan O, Bertholf RL. Monitoring point-of-care testing compliance. Clin Lab News. Feb 1, 2016. https://www.aacc.org/publications/cln/articles/2016/february/monitoring-point-of-care-testing-compliance.
- Nichols JH. Point of care testing. Clin Lab Med. 2007 Dec;27(4):893-908, viii
- Healthcare Financial Management Association: Health Care 2020: A series of reports examining how to prepare for major healthcare market trends over the coming years. Report 3 of 4: Consolidation. Fall 2016.
- CLIA website: http://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Downloads/HowObtainCLIACertificate.pdf.
- Lazarus IR, Chapman MW. ISO-style healthcare: designed to keep patients, practitioners and management safe. Becker’s Hospital Review. September 26, 2013. http://www.beckershospitalreview.com/hospital-management-administration/iso-style-healthcare-designed-to-keep-patients-practitioners-and-management-safe.html.
- ISO website. ISO 15189:2012 Medical laboratories—Requirements for quality and competence. https://www.iso.org/standard/56115.html.
- Gramz J, Koerte P, Stein D. Managing the challenges in point-of-care testing, an ecosystem approach. Point of Care. 2013;12(2):76-79.
Connie Mardis, MEd, serves as POC Value Ambassador for Siemens Healthineers.
Daniel C. Gundler serves as Director, Global Marketing-POC Informatics for Siemens Healthineers.