In October of 2016, the Department of Health and Human Services (DHHS) released the final Medicare Quality Payment Program (QPP) rule, which is a part of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). MACRA repealed the sustainable growth rate (SGR) formula that was used to determine Medicare reimbursements for physicians, and replaced it with the QPP framework, changing the way physicians are paid.
Value-based care and interoperability
While there is disagreement about repealing or replacing the Affordable Care Act (ACA), the Republican and Democratic parties both appear committed to patient-centered care with a strong focus on value and positive patient outcomes. As such, MACRA’s QPP rule passed with a strong bipartisan majority. Additionally, in line with other federal regulations, the Medicare QPP is a driving force for interoperability and a bigger push away from fee-for-service (FFS) toward value-based payment models. The QPP defines how providers will be reimbursed for quality and gives providers two options: the Merit-based Incentive Payment System (MIPS) or Advanced Alternative Payment Models (APMs). Only those who are already well into value-based reimbursements will qualify for APMS; therefore, the majority of providers will fall under MIPS.
Reimbursements for quality care
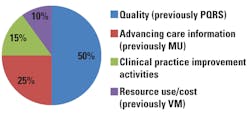
Intended to be implemented in stages, the QPP incentivizes providers to focus on population health management, preventive care, and quality. MIPS consolidates Meaningful Use (MU), the Physician Quality Reporting System (PQRS), and the Value-based Payment Modifier (VM). While hospitals will still be subject to MU Stage 3 in 2018, January 2017 marked the start date for MACRA reporting for eligible clinicians. MIPS-eligible clinicians will receive a composite performance score for four categories (based on 2017 data) that will determine their payment adjustment for 2019 (Figure 1):
- Quality (50 percent)
- Advancing care information (25 percent)
- Clinical practice improvement activities (15 percent)
- Resource use/cost (0 percent in 2017,
- 10 percent in 2018).1
PQRS, VM, and the Medicare EHR Incentive Program or MU have each played an important role in the beginning stages of quality measurement and reporting for Medicare. By aligning these programs within MACRA, CMS is consolidating requirements to reduce the reporting burden on clinicians.
Quality domains
Continued development and reporting of quality measures is important in the transition to value-based reimbursement models and patient-centered care. Several of the quality domains offer opportunities for laboratories to contribute. Included within the category of quality are outcomes measures for:
- Clinical care
- Safety
- Care coordination
- Patient and caregiver experience
- Population health and prevention
- Affordable care (overuse of clinical tests).1
Four ways labs can contribute
The laboratory information system (LIS) is part of an interoperable network of electronic data sources that feeds the EHR, resulting in a rich set of clinical data to use for quality measures. As a first step, labs can use their LIS to improve internal efficiency and promote rapid expedition of results that can impact quality scores. Second, LIS data can be tapped to help identify patients who, based on their lab results, are candidates for further preventive testing, and to provide data that supports population health measures. Third, and more important for today’s laboratory, the LIS can be used to track and support utilization management to ensure best use of resources. And last, laboratories are an integral part of the precision medicine plus companion diagnostics equation.
Efficiency & TAT
In today’s healthcare environment, particularly with Protecting Access to Medicare Act (PAMA) reimbursement cuts looming over labs, it is vital for labs to perform at top efficiency, producing fast, yet accurate, test results that can be used to diagnose and treat patients quickly. Today’s top LISs have perfected the integration of advanced decision support result evaluation rules that streamline nearly every aspect of testing. Decision-support rules automate and standardize tasks to increase efficiency, productivity, and error-proofing throughout the total testing process, and free staff for other complex duties. Two effective ways to improve a lab’s efficiency and value using decision-support rules are auto-verification of results and set-up of reflex testing cascades that allow it to provide the most appropriate testing and readily support utilization management efforts.
Population health and preventive services
Laboratories can also use their LIS to collect population-level data that can support the organization’s efforts toward population health management. Population health involves impacting the health and outcomes of a lab’s specific group of patients to promote better health. Measures in this quality domain involve determining if clinical services (such as lab testing) and preventive services are used in such a way that improvements in population health occur. Included in this domain are process measures that focus on prevention of disease by screening for early detection. Laboratories can use LIS data to determine patients for whom preventive testing would be beneficial based on evidence-based guidelines (e.g., identifying women eligible for HPV screening by age groups).
Utilization management
Tracking laboratory utilization management falls within the quality domains of affordable care and safety. Quality measures focus on lowering costs, reducing errors, and improving outcomes. The Centers for Medicare & Medicaid Services (CMS) considers utilization management a high priority within the QPP, and are collaborating with various stakeholders to adopt evidence-based practices for appropriate use.1 One such resource is the Choosing Wisely initiative of the American Board of Internal Medicine (ABIM) Foundation, which publishes evidence-based recommendations for the appropriate use of tests and procedures to reduce inappropriate use. Labs can use LIS data to track test utilization and identify unnecessary testing combinations. This can be part of ongoing feedback to providers to promote improved quality scores.
The Cures law
Another area of bipartisan agreement in Congress was the passage last December, by large majorities in both Houses, of the 21st Century Cures Act. The Cures law builds on the Precision Medicine Initiative and targets approximately $5 billion toward National Institutes of Health projects. Laboratories that serve a patient population that can benefit from companion diagnostic testing—testing to determine drug treatment efficacy—should consider the feasibility of including companion diagnostic testing on their menu.
To sum up
Medicare’s new QPP speaks to a continued focus on quality, preventive care, better utilization of services, and population health management. While much of the data and metrics used to support quality measures comes from the EHR, the LIS plays an important role as an information system that feeds the EHR and as an essential tool to help laboratories increase efficiency and monitor their internal utilization and productivity as it relates to quality measures.
Overall, there is a feeling of uncertainty as laboratories wait for the PAMA gavel to fall, and it is vital for labs to be part of making improvements in our healthcare system. Quality is being measured with clinical data, and labs handle a tremendous amount of that data. They can leverage the tools in their LIS to be part of promoting safety, population health, and preventive measures to support providers in improving patient outcomes.
REFERENCE
- Centers for Medicare & Medicaid Health Services Advisory Group, Inc. (2016, May 2). CMS quality measure development plan: Supporting the transition to the merit-based incentive payment system (MIPS) and alternative payment models (APMs). https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/Final-MDP.pdf.
Kim Futrell, BS, MT(ASCP), currently serves as Products Marketing Manager for Orchard Software. Kim has more than 20 years of laboratory management experience. Prior to joining Orchard in 2012, her role was as Operations Manager of a multispecialty physician’s office in North Carolina.
About the Author

Kim Futrell, MT (ASCP), MSHI
is the Products Marketing Manager at Orchard Software. Prior to joining Orchard in 2012, Futrell, who has a Master of Science in health informatics, worked as a lab manager for more than 20 years.