LIS of the future: supporting value-based measures for healthcare
Laboratory testing plays a major role in generating data needed to support effective and appropriate clinical decisions, providing information that aids in the prevention, diagnosis, treatment, and management of disease, as well as being an indicator for individual and population health. Despite this scale of importance, laboratory spending accounts for only 2.3% of U.S. healthcare costs and 2% of Medicare costs.1 As healthcare makes major shifts in response to recent legislation, appropriate use of laboratory testing becomes even more essential for achieving safe, effective, and efficient care for patients. Essential to this effort is the effective implementation of a mature, capable laboratory information system (LIS).
During the past 30 years, laboratory information systems have developed from simple systems designed to generate accurate reports to much more complex systems capable of tracking the entire laboratory workflow throughout all phases of the total testing process, including pre-analytical, analytical, and post-analytical phases of testing.1 Advances in information technology have facilitated major improvements in laboratory data management and interoperability, enabling healthcare facilities to link their LIS with electronic healthcare records (EHRs), hospital information systems (HISs), reference laboratories, practice management systems, and pharmacy databases to make improvements to our healthcare system. The future LIS will evolve from today’s systems to more mature quality solutions that enable pathologists and laboratorians to be an integral part of the care team with a clear focus on patient-centered care—healthcare that promotes wellness and prevention while simultaneously reducing spending.
External forces shaping LIS and EHR structure
We are in the midst of a complete overhaul of our healthcare system that will require a widespread culture change. There are so many factors shaping the future of our healthcare system that the alphabet soup of acronyms can be mind-boggling—and all of these forces are guiding the development of both the LIS and the EHR.
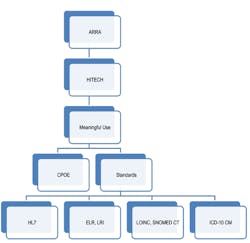
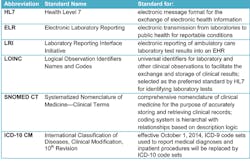
The health information technology (HIT) industry is awash with governmental regulations and compliance challenges (Figure 1). These include the Health Information Technology for Economic and Clinical Health Act (HITECH), part of the American Recovery and Reinvestment Act of 2009 (ARRA). HITECH oversees the Medicare and Medicaid EHR Incentive Program, which provides financial incentives for the Meaningful Use (MU) of certified EHR technology. Components of MU include Computerized Physician Order Entry (CPOE) and a plethora of standards (Figure 2).
Figure 1: Forces shaping LIS and EHR development
Figure 2: Standards list
Structured data for analytics. HIT systems of the future need interoperability so that data from different systems can be combined for effective business analytics. Essential to this is the use of structured, discrete data encoded with standardized vocabularies, such as LOINC (Logical Observation Identifiers Names and Codes) and SNOMED CT (Systematized Nomenclature of Medicine—Clinical Terms). This will allow disparate systems to communicate by ensuring data is structured in the same way, and will categorize and add meaning to terms to facilitate quick, clear understanding and retrievable data.
Additionally, ICD-9 codes will begin to disappear as we move to the more descriptive ICD-10 codes, enabling better utilization analysis and allocation of resources. However, IT systems will have to ramp up to support the more sophisticated coding system because along with the benefits of the advanced coding allowed by ICD-10 comes a much greater demand for hardware, software, implementation, and training and support. The Clinical Quality Measures, or CQMs, required by future stages of MU will need ICD-10-based data elements in order to facilitate the ability to study specific data about patient conditions and treatments. As accountable care environments begin to manage population health, the increased detail of ICD-10 will enable more accurate predictive modeling, which is paramount to more in-depth understanding of a population’s health status.2
New reimbursement models: based on outcomes
To build upon the HITECH foundation, in March 2010, the Patient Protection and Affordable Care Act (PPACA) was enacted—a comprehensive healthcare reform plan that will continue to evolve over the next decade. The PPACA stems from the Triple Aim vision that calls for simultaneous goals of improving patient experience, improving population health, and reducing per-capita healthcare costs.
Accountable Care Organizations (ACOs) and Patient-Centered Medical Homes (PCMHs) are examples of types of models that are being implemented to begin a shift toward payment models that reimburse for performance rather than procedure. Under these payment reform models, we are seeing common elements, such as risk-contracting and bundled payments that require a coordinated care mindset and tie provider reimbursements to improvements in quality measures and cost savings. ACOs become responsible for the overall health of their specific member population and thus benefit by determining ways to maintain the health of those patients and eliminate costly episodes of care. This is what is meant by population health management.
So the question becomes: how can the LIS and the EHR work together to provide the predictive data needed to support population health management? The EHR and the LIS have different but complementary tools that can be used to support the analytics needed by outcome-oriented organizations. As healthcare facilities tackle the improvement of the overall health of a population and enter risk-sharing payment models that require reporting of detailed analytics, they are turning to payors and HIT vendors for benchmarking analytics and patient-risk-stratification data. Data from the lab is an integral part of this process, and the LIS needs the interoperability to be able to share its clinical data.
What does the lab bring to the table?
Laboratory data is needed for screening, prompt diagnosis, treatment decisions, and monitoring and measuring effectiveness—all portions of the overall goal of improving the health of a population. Inefficiencies such as overutilization, redundant test orders, and lack of follow-up on lab results must be eliminated in order to achieve the cost saving and efficiency required by the new performance-based business models, and the LIS of the near future needs to have the functionality to support laboratory efforts to guide these initiatives.3
Laboratory data: the heart of healthcare decisions. One of the tools being used as healthcare organizations become responsible for the health of a specific patient population is risk stratification, or predictive modeling. Risk stratification involves using clinical data to make an educated forecast as to the likelihood a given patient will develop a certain medical condition. By combining lab data with claims and EHR data, patients can be given a risk-score that can proactively predict their healthcare needs.
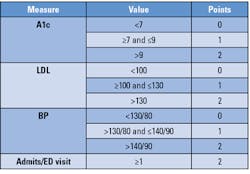
From this data collaboration, the care team can then recommend appropriate preventive and chronic care interventions. Placing a patient into a risk category can help with the development of a personalized care plan with the goal of helping each patient achieve his or her best health by preventing chronic disease, stabilizing certain chronic conditions, and preventing acceleration into a higher risk category that would lead to increased costs.4 Figure 3 depicts an example that is being used at LeHigh Valley Health Network in Pennsylvania that utilizes lab data in conjunction with other patient data to provide predictive risk scores for their patients.5
Figure 3: Risk score example from LeHigh Valley Health Network
Low risk = 0-2 points; Moderate risk = 3-5 points; High risk = 6-8 points
Role of the advanced LIS: analytics and efficiency. Our healthcare system has developed in fragmented silos of care, and this has created an inefficient system that causes poor utilization of lab tests. By using the LIS and the data within, labs can support triple-aim goals and make a positive impact at the point of order for diagnostic testing. This will ensure that the most appropriate and cost-conscious tests are selected. Laboratorians and pathologists can be instrumental in the collaborative efforts needed to track and monitor test utilization patterns and be involved in committees to develop and implement best-ordering guidelines and evidence-based testing algorithms or cascades that lead to optimum patient outcomes.
Widespread use and integration between EHRs and LISs are instrumental in supporting efforts to improve care coordination, reduce duplicate and redundant tests and procedures, and reward hospitals for keeping patients healthier. HIT systems give providers and hospitals the ability to better coordinate care, reduce errors and readmissions, reduce per capita costs, and make improvements to the overall health of the population.6 Laboratory professionals should be involved in monitoring test utilization practice and support that with metrics and LIS-driven testing cascades and algorithms.
The LIS of the near future will be able to eliminate many of the repetitive tasks currently performed by lab staff and provide predictive analytics at a glance. This can enable increased efficiency and reduced waste because clinical laboratory professionals are more available for the tasks that require human interpretation and hands-on procedures. Figure 4 lists attributes that should be considered as the LIS continues to evolve.
Figure 4: LIS of the future1
As healthcare advances are made, another consideration is how the LIS can support the Clinical Decision Support (CDS) rules required of EHRs by MU, in which data from the patient’s problem list, medication list, demographics, and laboratory results are compiled to generate automatic, electronic care suggestions in real time.7 The future LIS needs to be able to feed this CDS system by communicating flags, alerts, or other data such as historical test results in order to fulfill this MU requirement to support effective population heath management.
Point-of-care (POC) testing
As healthcare reform encourages a more proactive and patient-interactive stance, POC testing can offer substantial benefits because of its ready availability and quicker turnaround time, potentially reducing ER visits and hospital admissions. With millions of individuals being added to the insurance pool, a shortage of primary care providers, and retail clinics becoming involved in the business of healthcare, these are areas where POC testing can have a positive impact. As ACOs and Integrated Diagnostic Networks (IDNs) continue to develop, more testing will be performed outside of the core lab, in ambulatory settings and POC locations. It will become extremely important to coordinate this testing and ensure that all healthcare workers on the care team throughout the network have access to the data in order to make timely, optimal care decisions. And it will be important that these POC test results are captured by the EHR for quicker diagnosis and treatment, for billing, and so that data from POC testing can be included in analytics needed for risk stratification and population health statistics. An important question to consider is how to best integrate that POC test data. Does it route through the LIS? Straight to the EHR? The new business model may include the concept of increased POC testing; the lab of the future will be more pervasive, and that’s where POC can help fill in the gaps.
Outreach and integration
One of the biggest challenges facing laboratories providing outreach services is the need to seamlessly and affordably interface with many different EHRs simultaneously, combined with the fact that there are more than 1,000 EHR products listed on the Department of Health and Human Services certified product list. In order to be competitive, labs are forced to deal with a wide variety of EHR products that vary tremendously in their capability to send electronic lab orders.8 Additionally, many EHR vendors have a backlog of interface projects that slow progress and add to the challenge. Implementation of multiple EHR-LIS interfaces involving the setup of CPOE requires substantial efforts with regard to workflow, technical factors, and administrative needs, making it a complex endeavor. The LIS needs to be able to support effective and proactive management of each laboratory outreach client relationship.
Pharmacy integration. As healthcare’s collective focus turns to proactively managing the overall health of populations, interoperability between ancillary healthcare systems becomes mandatory, and one of the most beneficial electronic collaborations occurs between the laboratory and the pharmacy. The advantages of patient information exchange between the pharmacy and the lab include the following:
- In companion diagnostics, there is a tight linkage between medication administration and a specific molecular test result.
- Pharmacists can determine the most effective and cost-conscious antibiotic treatment based on the microbial findings from the laboratory.
- Monitoring of long-term medication usage (such as anticoagulants) to reduce adverse drug events (ADEs) can be improved by a collaborative effort between laboratorians and pharmacists.9
Just as pharmacists can be more proactive in their prescription recommendations to the physician because of access to lab data, labs can benefit from connectivity to patients’ medication lists to interpret certain tests by using a combination of the drug testing recommendations and delta check data for more informed and more efficient test interpretations.
Added challenges
Advances in molecular and genetics. Certain specialty areas bring increased challenges to the growth and development of the LIS. Physicians are faced with ever-expanding laboratory test menus increasing in complexity due to growth in the use of molecular and genetic testing. Anticipated to continue to increase, molecular testing has grown steadily over the last decade, at a rate averaging 15% annually, as compared to an average growth in clinical lab testing of around 5%.10
The volume and complexity of data generated by genetic and molecular tests will require that LISs be capable of storing and retrieving large amounts of data. Additionally, molecular and genetic testing bring other unique LIS challenges including specialized nomenclature, image import and export, karyotype concepts, integration of images, advanced algorithms and statistics, and sequence and fragment analysis instrument interfaces.1 Most important, molecular techniques and instrumentation can change at a rapid pace, making the need for the LIS to be scalable and adaptable one of extreme importance. The LIS will have to have the agility to adapt to these emerging technologies quickly.
Culture change, leadership, and patient engagement. The future will require that the LIS and the EHR work together to absorb information from pathology, molecular, radiology, pharmacy, and other ancillaries, and then feed that data to a computing environment capable of analyzing the patient symptoms, along with the clinical data and any applicable research data, and presenting probable diagnoses and treatment options to assist providers in more efficient patient care.2 Innovative healthcare organizations such as Memorial Sloan Kettering and WESTMED are already working with supercomputer IBM Watson to provide their teams with better information and promote advanced decision-making.11,12
The transformation of our healthcare system is well under way and is beginning to demonstrate the merit of a focus on coordinated patient care and outcomes-based reimbursements. Laboratory leaders and physician leaders must synchronize goals and work together to determine how to best utilize both the LIS and the EHR to their full potential, combining the unique strengths of the two systems to maximize efficiency and reduce costs and result in improved patient outcomes and satisfaction.
The collective healthcare goal is to use technology to care for populations based on their disease-specific needs in a proactive and patient-interactive manner.3 The success of healthcare reform will require health information technology to provide and support interoperability across many clinical data systems. Because up to 80% of clinical decisions are made based on laboratory data—data that is stored in the LIS—a robust, configurable LIS can play a central role in supporting laboratorians in their efforts to continue making improvements to the healthcare system. Laboratory leaders need to be thinking of where the lab fits in the big picture of a value-based healthcare system and how labs can maximize their LIS to best support the needs of their healthcare organization and the patients they serve.
References
- The Lewin Group. Laboratory Medicine: A National Status Report. May 2008. www.futurelabmedicine.org/pdfs/2007%20status%20report%20laboratory_medicine_-_a_national_status_report_from_the_lewin_group_updated_2008-9.pdf. Accessed October 23, 2013.
- C. Natale. How ICD-10 coding can affect patient care. Healthcare IT News. November 14, 2012. www.icd10watch.com/blog/how-icd-10-coding-can-affect-patient-care. Accessed October 23, 2013.
- Futrell K. The EHR-LIS nexus. MLO. 2013;45(5);42-46.
- AAFP. Risk-stratified care management. www.aafp.org/practice-management/pcmh/initiatives/cpci/rscm.html. Accessed October 23, 2013.
- Berra A. Risk-stratifying patients—two example approaches. The Advisory Board Company. April 4, 2011. www.advisory.com/Research/Health-Care-Advisory-Board/Blogs/The-Blueprint/2011/04/Risk-Stratifying-Patients-Two-Example-Approaches. Accessed October 23, 2013.
- Doctors and hospitals’ use of HIT more than doubles since 2012 [news release]. US Dept of Health & Human Services; May 22, 2013. www.hhs.gov/news/press/2013pres/05/20130522a.html. Accessed October 23, 2013.
- Elhanan MD, Fry G, Fry M. Connecting Labs to Physicians EHRs: Effective Strategies for Labs to Add Value. Dark Daily Laboratory and Pathology News. 2011. www.darkdaily.com/white-papers/connecting-labs-to-physicians-electronic-health-records-effective-strategies-for-laboratories-to-add-value-42011#Form. Accessed October 23, 2013.
- HealthIT.gov. Certified Health IT Product List (CHPL). www.healthit.gov/policy-researchers-implementers/certified-health-it-product-list-chpl. Accessed October 23, 2013.
- Malone B. The emergence of lab-pharmacy partnerships. Clin Lab News. 2012;38(5). www.aacc.org/publications/cln/2012/may/Pages/LabPharmacyPartnerships.aspx. Accessed October 23, 2013.
- UnitedHealth Center for Healthcare Reform & Modernization. Personalized Medicine: Trends and prospects for the new science of genetic testing and molecular diagnostics. March 2012. www.unitedhealthgroup.com/~/media/uhg/pdf/2012/unh-working-paper-7.ashx. Accessed October 23, 2013.
- Memorial Sloan-Kettering’s collaboration with IBM Watson featured on CBS this morning [news release]. Memorial Sloan-Kettering Cancer Center; July 9, 2013. www.mskcc.org/blog/msk-s-collaboration-ibm-watson-featured-cbs-morning. Accessed October 23, 2013.
- Schwartz SA. Prescription for information overload. The Atlantic. April 25, 2013. www.theatlantic.com/sponsored/ibm-watson/archive/2013/04/prescription-for-information-overload/275288/. Accessed October 23, 2013.
About the Author

Kim Futrell, MT (ASCP), MSHI
is the Products Marketing Manager at Orchard Software. Prior to joining Orchard in 2012, Futrell, who has a Master of Science in health informatics, worked as a lab manager for more than 20 years.