Vitamin D has become one of the most widely discussed and intensely scrutinized supplements in recent history. The renewed interest is due in large part to the startling prevalence of vitamin D deficiency worldwide and the proliferation of articles linking deficiency to multiple clinical conditions other than bone health.
While recommendations for adequate blood levels and dietary intake amounts continue to be refined, it is clear that millions of Americans—from healthy-looking infants to the elderly, and many others in between—are deficient in this essential nutrient.
Not surprisingly, the clinical fascination with vitamin D has produced a corresponding rise in demand for associated laboratory tests. As new assays surface to address that need, it is critical for laboratorians to understand why they should test, what they should test for, and what criteria they should use to determine which test assay and platform best meet their needs.
The critical role of vitamin D
Vitamin D is a naturally occurring, biologically inert hormone precursor that exists in two primary forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Both forms are converted in the liver to the body’s main storage form of vitamin D, known as calcidiol, and then in the kidneys to the physiologically active form, or calcitriol.
In this final form, vitamin D acts as a hormone. Its main biologic function is to maintain serum calcium and phosphorus concentrations within the normal range by enhancing the efficiency of the small intestine in absorbing these minerals from the diet. The interaction of calcitriol with vitamin D receptors increases the efficiency of intestinal calcium absorption from only 10% or 15% to 30% or 40% and phosphorus absorption from about 60% to approximately 80%.1
When dietary intake is inadequate to satisfy the body’s calcium requirement, vitamin D facilitates increased calcium reabsorption in the kidney and works with parathyroid hormone (PTH) to mobilize calcium stores from the bone, effectively increasing serum levels of calcium.2
Because of its role in maintaining calcium homeostasis, vitamin D is essential to overall bone health, promoting healthy growth and remodeling. An insufficiency leads to thin, brittle or misshapen bones and can contribute to rickets in children, a disease characterized by a failure of bone tissue to properly mineralize, resulting in soft bones and skeletal deformities. In adults, vitamin D insufficiency leads to weak bones and osteomalacia, a disease that weakens bones and can cause them to break more easily. Along with calcium, vitamin D also helps prevent osteoporosis in older adults.3,4
Vitamin D has a number of other physiological roles, including maintaining muscle strength, modulating immune function, regulating cellular differentiation, and reducing inflammation. A growing body of research also suggests that vitamin D might play a role in the prevention and treatment of a number of diseases, including type 1 and type 2 diabetes, hypertension, glucose intolerance, multiple sclerosis, and other medical conditions, including cancer. However, current literature does not support a role for vitamin D in reducing the risk of cancer.4
Primary sources
The major source of vitamin D for humans is the sun. Vitamin D3 can be synthesized in the skin upon exposure to ultraviolet-B (UVB) radiation from sunlight, and thus sun exposure accounts for about 80% to 90% of vitamin D for most people. According to Mayo Clinic, a single exposure to summer sun in a bathing suit for 20 minutes produces the equivalent of 15,000 to 20,000 IU of vitamin D3.5
Vitamin D can also be obtained from the diet, in the form of either vitamin D2 or D3. While very few foods naturally contain vitamin D, fatty fish (such as salmon, tuna, and mackerel) and fish liver oils are among the best sources. Small amounts of vitamin D are also found in beef liver, cheese, and egg yolks. These foods primarily contain vitamin D3. Some mushrooms provide vitamin D2 in variable amounts.3
Most Americans receive the bulk of their dietary vitamin D from supplements or fortified foods, such as milk, ready-to-eat breakfast cereals, orange juice, and yogurt.4 In these sources, vitamin D is available in both D2 and D3 forms, although foods and over-the-counter supplements sold in the United States today are typically fortified with vitamin D3 rather than vitamin D2.3 However, preparations of vitamin D for prescription use in North America are still in the form of vitamin D2.6 There is some controversy regarding whether vitamin D2 is as efficacious as vitamin D3.7,8
Mechanisms of metabolism
Because vitamin D obtained from sun exposure, food, and supplements is biologically inert, it must undergo two processes in the body for activation. The first occurs in the liver, where it is converted to 25-hydroxyvitamin D [25(OH)D], also known as calcidiol.4 This prehormone is the body’s main storage form of vitamin D, and the amount of calcidiol available to the body is what determines vitamin D status. Guidelines for recommended vitamin D levels are referring to blood calcidiol levels.
Then, mostly bound to vitamin D binding protein, the 25(OH)D is secreted into blood plasma where, because of its relatively long half-life of two to three weeks, it serves as a reservoir for further hydroxylation.9
A second hydroxylation occurs primarily in the kidney, where 25(OH)D is converted to the physiologically active form 1,25-dihydroxyvitamin D [1,25(OH)2D], known as calcitriol, a potent steroid hormone.4 The kidney secretes calcitriol into circulation, again bound to vitamin D binding protein, where it travels to tissues involved in the regulation of calcium and phosphorus supply, namely intestine, bone, parathyroid glands, and the kidney itself. Once in circulation, the half-life of calcitriol is very short compared to that of calcidiol—only about four to six hours.9 Blood calcitriol levels are not good indicators of the amount of vitamin D and should not be used to determine sufficiency or deficiency.
Defining vitamin D deficiency
In the past, vitamin D deficiency was identified by a physical rather than a biochemical manifestation—namely, the presence of bone disease, either rickets or the adult equivalent, osteomalacia. But clinical symptoms of vitamin D deficiency can include chronic, nonspecific musculoskeletal pain, weakness, and fatigue that is non-specific as to age, mobility, sex, or ethnic group.
Today, serum concentration of 25(OH)D (calcidiol) is the best indicator of vitamin D status, and it allows for the detection and monitoring of vitamin D deficiency. It reflects vitamin D produced by the skin (from sunlight) and obtained from food and supplements. Calcidiol functions as a biomarker of exposure, but it is not clear to what extent 25(OH)D levels also serve as a biomarker of effect (i.e., relating to health status or outcomes).
The serum level of 25(OH)D that is defined as vitamin D deficiency remains somewhat controversial. There is considerable discussion of the concentrations associated with deficiency, adequacy for bone health, and optimal overall health, and cut points have not been developed by a scientific consensus process. Most experts agree with the Endocrine Society guidelines, which state that deficiency should be defined as a vitamin D level of 10 The Endocrine Society further defines insufficiency as 21-29 ng/mL. Similarly, the U.S. National Kidney Foundation considers levels Figure 1).11 The preferred level for vitamin D now recommended by many experts is ≥30 ng/mL. This is in line with the U.S. National Osteoporosis Foundation recommendation of levels ≥30 ng/mL to protect bone health.12
Based on its review of data of vitamin D needs, a committee of the Institute of Medicine (IOM) concluded that: (1) persons are at risk of vitamin D deficiency at serum 25(OH)D concentrations 50 ng/mL are associated with potential adverse effects (Figure 2).4
The incongruity of the guidelines from various organizations can be explained by the differences in their intent. The goal of the Endocrine Society guideline was to provide guidance for clinicians to maximize the health of individual patients, rather than to make recommendations for normal healthy populations, as covered by the IOM report.13
Screening and risk factors
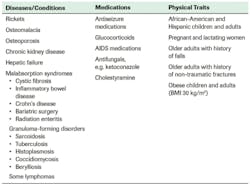
Accordingly, the Endocrine Society recommends vitamin D screening for individuals at risk for deficiency but not for general population screening for those who are not at risk (Figure 3).10
However, the number of individuals at risk is significant—and growing. About one-third of Americans have vitamin D levels that are less than adequate for bone and overall health in healthy individuals, with more than 23 million at risk of vitamin D deficiency and/or inadequacy based on IOM categorization (serum 25(OH)D 2
Since 1994, the number of Americans with 25(OH)D levels under 30 ng/mL (the National Kidney Foundation threshold for insufficiency/deficiency) has doubled. The downward trend in vitamin D levels is associated with a decline in consumption of milk that is fortified with vitamin D, decreased sun exposure and increased use of sunscreen, and an increase in body mass index (BMI) worldwide. (A rise in BMI causes more vitamin D to be sequestered in subcutaneous fat and not released into the circulation.)
Assay and platform considerations
As research links vitamin D deficiency to disease states other than bone disorders, the volume of vitamin D testing continues to increase throughout the world. The global testing market is expected to grow at a compound annual growth rate of 33.5% through 2014. New methodologies have been developed to help labs meet the increased demand for testing, including both immunoassay and protein binding assays, some of which run on automated platforms.
As a result, many laboratories are considering bringing vitamin D testing in house. There are several factors that a lab should assess in order to evaluate ROI and determine which platform and assay best meets its needs: (1) the cost of the equipment, especially if the testing method requires a dedicated system; (2) the complexity of the testing method in terms of staff time and proficiency requirements; and (3) the efficiency of the testing method and the relative balance of throughput with demand.
Another key consideration is what form(s) of vitamin D the test should recognize. For assessment of vitamin D status in patients at risk for deficiency, Endocrine Society guidelines recommend a serum circulating 25-hydroxyvitamin D [25(OH)D] level measured by a reliable assay.10 Otherwise known as a total vitamin D assay, a 25(OH)D assay should be able to recognize both vitamin D2 and D3 metabolites. With the use of vitamin D2 supplements declining, 99% of all patients tested will have no circulating 25(OH)D2, and it will likely only present if the patient is taking Drisdol (prescription D2). However, since Drisdol is also the sole therapeutic form of vitamin D in the U.S., it is important for the assay to be able to detect total circulating 25(OH)D—both 25(OH)D2 and 25(OH)D3.14
The results reported for a total vitamin D assay may differ based on the method used. Immunoassay methods report a single total vitamin D result; high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS) methods report vitamin D2 and vitamin D3 values separately and the two values are added together to obtain the total vitamin D value. Current scientific literature does not indicate that there is a clinical advantage to the latter approach, as it is the total 25(OH)D value that drives a clinician’s decision to treat.14
Standardization, certification, and proficiency testing
While LC-MS and immunoassay are the two most common methods used for vitamin D testing today, considerable variability exists among the assays available and among laboratories that conduct the analyses, due to the lack of standardization. In order to address this issue, the NIH Office of Dietary Supplements has established the Vitamin D Standardization Program (VDPS) in collaboration with the Centers for Disease Control and Prevention, the National Institute for Standards and Technology (NIST) and Ghent University. The aim of the program is to standardize the laboratory measurement of vitamin D status in national health surveys worldwide by linking them to the NIST reference measurement procedure (RMP).15 The American Association for Clinical Chemistry (AACC) and the College of American Pathologists (CAP) are among the collaborative partners in the project.
The first step in developing a vitamin D standardization protocol is to develop a reference system in order to establish a metrological chain of traceability from an assay to the reference method procedure. This has already been done with the NIST and Ghent labs using LC-MS/MS.
A number of manufacturers are enrolled in inter-laboratory comparison and commutability studies, enabling their vitamin D assays to be standardized to the NIST-Ghent RMP. So one of the first criteria the lab should consider in evaluating a vitamin D assay is whether it is standardized against LC-MS/MS that is traceable to the National Institutes of Standards and Technology standard.
In addition, the CDC has developed a certification program that will monitor and certify the accuracy and precision of vitamin D test methods on a yearly basis, similar to the certification process for lipids, testosterone, and HbA1c. Once the test method is standardized to the RMP, participants can submit results from four quarterly challenges, which involve 10 blinded, single donor serum samples per challenge. These four challenges are used to determine whether the method can meet an imprecision goal of a CV of ≤10% and bias of ≤5%. These criteria are based on data on biological variability for vitamin D. So the laboratory may want to confirm whether the test manufacturer is participating in this CDC certification program to verify end user performance for its vitamin D assay.
Finally, the laboratory should consider using a proficiency testing provider offering accuracy-based reference targets assigned by LC-MS/MS traceable to the NIST standard. The rationale for using accuracy-based as opposed to peer group-based surveys is that assay results are used for diagnosis and treatment. Survey samples should be minimally processed human serum to avoid “matrix effects.” And consistent with the standardization discussion above, target values should be assigned by a reference laboratory using a recognized RMP, such as the one developed by NIST, CDC, and Ghent University. Examples of proficiency testing providers meeting these criteria include the College of American Pathologists (CAP) and the Vitamin D External Quality Assessment Scheme (DEQAS).
From a broad perspective, these standardization, certification, and proficiency testing factors should be considered in conjunction with a variety of other criteria in order for the lab to determine which assay and platform provide the best vitamin D testing solution. Carefully balancng assay performance requirements with capital costs, staff capabilities, and throughput needs will enable a lab to meet demand and turnaround time expectations, provide enhanced medical value to clinicians, and strengthen its financial position as the volume of vitamin D testing continues to rise.
Andrea M. Rose, PhD, MBA, is a Senior Clinical Support Consultant for Roche Diagnostics in Indianapolis, IN.
REFERENCES
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
- 2nd National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population. www.cdc.gov/nutritionreport/pdf/Fat.pdf. Accessed March 24, 2013.
- Linus Pauling Institute. Research Newsletter Spring/Summer 2008. http://lpi.oregonstate.edu/ss08/vitamind.html. Accessed March 24, 2013.
- Dietary Supplement Fact Sheet: Vitamin D. Office of Dietary Supplements, National Institutes of Health. http://ods.od.nih.gov/factsheets/Vitamind-HealthProfessional. Accessed March 2013.
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
- Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-Hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93(3):677–681.
- Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95(2):471-478.
- Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84(4):694-697.
- Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88(2):500S-506S.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
- National Kidney Foundation. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Children with Chronic Kidney Disease. New York: National Kidney Foundation; 2005.
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010.
- Holick MF, Binkley NC, Bishoff-Ferrari HA, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97(4):1153-1158.
- Hollis BW. Measuring 25 hydroxyvitamin D in a clinical environment: challenges and needs. Am J Clin Nutri. 2008;88(2):507S-510S.
- Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: National surveys and the problem of standardization. Scand J Clin & Lab Invest. 2012;72(243):32-40.