To earn CEUs, see current test at
www.mlo-online.com
under the CE Tests tab. The September test covers all articles in this
section, except the product announcement.
LEARNING OBJECTIVES
Upon completion of this article, the
reader will be able to:
- Describe recent advances in fetal lung-maturity testing.
- Describe issues related to hCG and neo-natal bilirubin testing.
- Describe limitation protocols for blood collection from neonates.
- Describe information related to non-invasive pre-natal blood tests.
- Identify screening tests performed on U.S. births.
Scientists seeking answers for fetuses and infants
Edited by Carren Bersch, Editor
MLO invited David G. Grenache, PhD,
D(ABCC), and Brad S. Karon, MD, PhD, to respond to questions regarding
the topics of the presentations they made at AACC 2009 in Chicago
recently. Those presentations are available on DVD through AACC at
http://aacc.eventmediastore.com. Dr. Grenache is assistant
professor of Pathology at the University of Utah School of Medicine, and
medical director of Special Chemistry at ARUP Laboratories, both in Salt
Lake City, UT. Dr. Karon, is assistant professor of Laboratory Medicine
and Pathology, and director of the Hospital Clinical Laboratories,
Point-of-Care Testing, and Phlebotomy Services at Mayo Clinic in
Rochester, MN.
MLO: Briefly explain how you became
interested in studying fetal lung-maturity testing.
David G. Grenache, PhD: When I was
a post-doctoral fellow in clinical chemistry the laboratory
called to tell me they had accidently centrifuged an amniotic
fluid specimen that had been submitted to the lab for a fetal
lung-maturity (FLM) test. As these specimens are not to be
centrifuged prior to analysis, the specimen was clearly
compromised but, being that it was amniotic fluid, it was not
easily re-collected. I began to wonder what effect
centrifugation and resuspension had on the result of the FLM
test, so I set about doing a series of investigations that
examined not only the centrifugation effect but also other
preanalytical factors that might affect the test. The results of
this study were published in Clinical Chemistry.
MLO: How often do changes occur
in fetal lung-maturity testing? Based on your experience in fetal
lung-maturity testing, what changes in equipment or tests do you believe
might be implemented in the near future that would affect laboratory
testing or testing interpretation?
Grenache: The first test of
fetal lung maturity was the L/S (lecithin/sphingomyelin) ratio that was
introduced in 1971. It was an important test because, until then, there
were no tests to detect lung maturity.
The loss of this test to the laboratory community
would have profound effects for FLM testing. Labs that currently offer
the test may need to find a replacement for it or begin sending FLM test
requests to a referral lab. Since physicians expect FLM tests results to
be returned quickly (<12 hours), sending specimens out is not an ideal
solution. When considering a replacement test, there are a few options.
If laboratories do not currently do the L/S ratio — and only ~15% of
labs do — they might consider doing so, but the test is technically
difficult to perform, requires considerable expertise, and is imprecise.
The rapid detection of PG (phosphatidylglycerol) is an option, but PG is
a late marker of lung maturity and the result is qualitative, so it is
not an appropriate replacement for the quantitative S/A ratio. That
really only leaves the LBC test and, in my opinion, that is probably the
most suitable and practical replacement test.
MLO:What do you believe
is the most important single thing a laboratory scientist should
know about fetal lung-maturity testing in terms of the most
up-to-date information you have derived from your studies — and
why? How does this impact the medical laboratory in light of its
current tests and procedures?
2001;97:318), which suggested a maturity cutoff of 50,000 LBC/uL, but Ann M. Gronowski, PhD, an associate professor at the Department of Pathology and Immunology Washington University School of Medicine in St. Louis, MO, and her colleagues have shown that this cutoff is not appropriate for all brands of cell counters (Clin Chem. 2003;49:995-997).
Labs wishing to offer the LBC will obviously need
to do a thorough validation, because it is a lab-developed test, and the
issue of a maturity cutoff is very important aspect of that validation.
Fortunately, the cutoff recommended in the guidelines was derived from a
Beckman-Coulter (BC) cell counter, and studies indicate that concordance
among all BC-brand counters, regardless of model, are quite good. If a
lab is using a BC-type counter, then the 50K maturity cutoff is likely
appropriate.
MLO: If not in fetal
lung-maturity testing, in what other area(s) of pre-natal
testing do you foresee improvements in equipment or changes in
tests that would affect laboratory testing or testing
interpretation?
Grenache: Various issues
surrounding hCG (human chorionic gonadotropin) tests, both
quantitative and qualitative, have been getting a lot of
attention lately. Like most immunoassays for heterogeneous
molecules, hCG assays are not standardized. Recent work by
Sturgeon and colleagues has demonstrated the variability of
results between hCG assays (Clin Chem. 2009;55:1484) —
and also in an editorial I co-authored with Dr. Gronowski (Clin
Chem. 2009;55:1447) — by identifying the analytical
specificity of hCG assays. Similar studies in my lab have
confirmed Sturgeon's report, and Dr. Gronowki and my report is
currently under review.
I foresee that there will be improvements in hCG
assay standardization, and this will certainly improve the clinical
utility of hCG assays, not so much for pregnancy diagnosis but for
oncology applications (which, in the United States, are off-label uses)
where serial measurements of hCG are common.
As a side note, work on hCG assay standardization
was begun in 1994 when the International Federation of Clinical
Chemistry and Laboratory Medicine established a group to work on that
issue. If successful, it would serve as a model for other protein
hormones as well.
The qualitative detection of hCG in urine-using
rapid point-of-care (POC) tests are also in need of improvement. A few
years ago, I reported that qualitative hCG tests could detect hCG
variants that were unexpected (Clin Chem. 2007;53:989). By
design, these tests should only detect the dimeric hormone; but,
surprisingly, some of them can detect non-intact variants. A recent
report from Dr. Gronowski and colleagues has now shown that
false-negative results can occur when the urine sample contains high
concentrations of an hCG variant called the “beta core fragment,” which
is the major hCG variant in urine from the fifth week of gestation to
term. False-negative hCG results are particularly dangerous because they
suggest the absence of pregnancy, and the mother may undergo a procedure
or treatment that is potentially harmful to the fetus. Manufacturers of
POC hCG tests need to address this issue to prevent this type of
interference from occurring.
MLO: Briefly explain how you
became interested in neo-natal studies, particularly hyperbilirubinemia.
Brad Karon, MD, PhD: I received an e-mail
from my colleague, Walter J. Cook, MD, in Pediatrics who was interested
in the idea of transcutaneous bilirubin-screening but had no idea how to
conduct a device or method validation. Our initial communication has now
led to two clinical studies and a clinical protocol in our nursery for
transcutaneous bilirubin screening. It has been an enjoyable and
rewarding collaboration with Dr. Cook. In addition, it is satisfying to
see a new clinical protocol in our large practice being developed
directly from data that we obtained together.
MLO: What do you believe is the most
important single thing a laboratory scientist should know about that in
terms of the most up-to-date information you have — and why?
Karon: We need to continue to raise the
level of awareness about the risk of kernicterus in neonates if we do
not employ systematic risk-assessment protocols for hyperbilirubinemia
in our hospitals and nurseries. For laboratorians, the most important
message is probably to find a way to communicate bilirubin values,
whether serum/plasma or transcutaneous, in ways that allow clinicians to
interpret them with regard to the infant's post-natal age in hours.
Age-specific nomograms or protocols exist or can be developed for both
laboratory and transcutaneous bilirubin — and we need to collaborate
with our clinician colleagues to make sure we give them enough
information to do this.
MLO: How often do changes occur
in this area of neo-natal testing? Based on your experience, what
changes in equipment or tests do you believe might be implemented in the
near future that would affect laboratory testing or testing
interpretation?
MLO: In what other area(s) of neo-natal
testing do you foresee improvements in equipment or changes in tests
that would affect laboratory testing or testing interpretation?
Karon: There are several groups interested
in looking at genetic determinants of bilirubin rise after birth,
something that could add to the tools already available to predict which
infants will need close monitoring or treatment for hyperbilirubinemia.
Right now, it is not clear whether better tests or better systems are
necessary to prevent kernicterus; though, if I had to guess, I would say
better systems are needed.
MLO: In the “history” or “timeline” of
neo-natal testing, when did the particular subject matter that commands
your attention initially arise; and where, in terms of importance in
that timeline, does your particular interest lie?
Karon: Kernicterus has been around for
centuries, though interest in this waned significantly in the 1970s and
1980s after the routine introduction of RhoGAM to prevent
alloimmunization of Rh-negative mothers. Interest in kernicterus
re-emerged in 2004 with the publication of new guidelines by the
American Association of Pediatrics (AAP). The AAP guidelines basically
pointed out that with shortened maternal and, thus, infant, admissions,
healthcare systems had not produced effective ways to evaluate infants
for the risk of dangerously high bilirubin levels that might develop
after discharge from the nursery. My interest developed in 2004 and
beyond with the new recommendations.
By Dennis Ernst, MT(ASCP)
Concern over excessive volumes of blood withdrawn
from newborns and infants is prompting facilities to adopt policies to
monitor total blood volumes that reduce the chance of phlebotomy-induced
(i.e., iatrogenic) anemia. Removing as little as 10 mL of blood from a
newborn can result in up to a 10% depletion of the infant's total blood
volume.1 Because newborns have between 80 mL and 110 mL of
blood per kilogram of body weight, those subject to multiple sampling
during the first week of birth (e.g., severely jaundiced newborns) are
especially vulnerable to complications.1
In one study, researchers concluded that phlebotomy
overdraws were responsible for up to 15% of the packed-cell transfusions
given to very low birth rate infants.2 An article published in
Clinical Leadership & Management Review reports of a study conducted in
Denmark that showed blood losses from diagnostic sampling constitute up to
45% of the total blood volume of infants studied.3
especially for premature infants and neonates, continues to frustrate
phlebotomists and their managers who want to minimize the impact of the
procedure on those most susceptible to phlebotomy-induced anemia.
Further, a study reported in Laboratory Medicine
shows that patients lose 4 mg of iron for every 10-mL tube of blood drawn.4
Therefore, not only do newborns and infants suffer from red blood-cell
depletion due to multiple sampling, but [also] the risk of developing iron
deficiency over time increases dramatically, [thus] increasing the impact of
blood-volume depletion.
According to the Clinical and Laboratory Standards
Institute's document H3-A4, Procedures for the Collection of Diagnostic
Blood Specimens by Venipuncture, “A mechanism should be in place to monitor
the amount of blood drawn for pediatric and critically ill patients, in
order to avoid phlebotomy-induced anemia.”5
The College of American Pathologists prompts volume
considerations in its accreditation checklist by asking a question about the
lab's review of “phlebotomy practices to minimize unnecessarily large blood
draw volumes.”3
Two publications suggest limitations on the volumes
of blood to be collected from pediatric patients. Phlebotomy Handbook
contains a chart that places limits on quantities withdrawn both per
collection and per admission on pediatric patients from six to 100 pounds
(2.7 kg to 45.5 kg). According to the authors, the recommended limit for an
infant who weighs between six and eight pounds (2.7kg to 3.6 kg) is 2.5 mL
per draw and 23 mL per hospital stay, up to one month.
A chart published in the CLSI Document H4-A6 shows
that 10 mL of blood constitutes 7.9% of the total blood volume in a
1.1-pound premature infant.6 But guidance in the literature stops
there, leaving it up to phlebotomists and their managers to devise their own
tracking system and develop protocols when their limits are met.
researchers so that laboratories can continue to provide critical test
results without threatening the already-fragile physiologies of neonates
and geriatrics.
Several sources provide assistance in establishing
blood-sampling limits based on body weight and on estimating circulating
erythrocyte volumes. For adults, blood volume can be calculated by applying
the general assumption that a patient's average blood volume is equal to 70
mL per kilogram of weight.3 Full-term neonates have a blood
volume of 80mL/kg to 110 mL/kg, while the volume for premature infants is
115 mL/kg.1
Using these statistics, a patient's circulating
erythrocyte volume can be calculated by multiplying the blood volume with
the hematocrit. For example, a full-term infant weighing 2.7kg (six pounds)
has an estimated total blood volume of 229 mL. If the infant's hematocrit is
55%, the erythrocyte volume is 126 mL (229 mL x .55).
If the facility caring for the infant adopts a policy
not to withdraw more than 7% of any patient's total erythrocyte volume in
any 24-hour period (arbitrary), then a calculation of the maximum allowable
whole blood volume that can be removed from the infant without extracting
more than 7% of the RBCs can be made.
Monitoring the volume of blood withdrawn as CLSI
recommends can be accomplished with a log sheet attached to the infant's
chart upon which phlebotomists and nurses can record volumes withdrawn or by
recording volumes in the laboratory records. Regardless of the approach, all
mechanisms have the potential to breakdown if all who collect specimens in
the facility do not comply.
Even with full compliance, however, tracking serves
no purpose unless limits are established on volumes sampled, and a protocol
is adopted and implemented when those limits are met or exceeded. Because no
current guideline exists in the literature, such a protocol takes a
coordinated effort between the laboratory, nursing, and medical staff, and
must begin with thorough research on the current thinking about newborn and
premature-infant blood volumes and the degree of blood loss the facility is
willing to establish as a limit.
This lack of clear-cut standards on maximum sampling
volumes, especially for premature infants and neonates, continues to
frustrate phlebotomists and their managers who want to minimize the impact
of the procedure on those most susceptible to phlebotomy-induced anemia.
More guidance is clearly needed from pediatricians
and researchers so that laboratories can continue to provide critical test
results without threatening the already-fragile physiologies of neonates and
geriatrics. Until standards emerge, facilities will continue to face the
challenge of setting their own limits on sampling volumes.
Reprinted with kind permission from Dennis J. Ernst,
MT(ASCP), the director of the Center for Phlebotomy Education in Corydon,
IN, and MLO editorial advisory board member.
References
- Becan-McBride K, Garza D, Phlebotomy Handbook. Upper Saddle River,
NJ: Pearson; 2010. - Lin J, Strauss R, Kulhavy J, Johnson K, Zimmerman M, et al.
Phlebotomy overdraw in the neonatal intensive care nursery.
Pediatrics. 2000;106(2). - McPherson R. Blood sample volumes: emerging trends in clinical
practice and laboratory medicine. Clin Leadership Mgmt Rev.
2001;Jan/Feb:3-10. - Q&A. Blood volumes needed for common tests. Lab Med.
2001;4(2):187. - Clinical and Laboratory Standards Institute. Procedures for the
Collection of Diagnostic Blood Specimens by Venipuncture; Approved
Standard—Sixth Edition. Wayne, PA: CLSI; 2007. CLSI Document H3-A6.
Clinical and Laboratory Standards Institute. Procedures and Devices for
the Collection of Diagnostic Capillary Blood Specimens; Approved
Standard—Sixth Edition. Wayne, PA: CLSI; 2008. CLSI Document H04-A6.
Creamatocrit testing
Mother's milk is the preferred feeding for
low-weight, premature infants because of its unmatched nutrition,
immunological, and anti-inflammatory properties. Because the caloric
density of mother's milk varies greatly, neo-natal intensive care units
are performing creamatocrit testing in order to measure the milk's fat
content and estimate the calories. Not only does this test provide a
baseline of what the infant is getting, certain processes can be
employed to raise the fat and calorie content, if desired. These
processes include determining the time a mother must pump in order to
express the calorie-dense hindmilk, capturing the lower-volume,
higher-calorie milk that generally occurs late in the day, correcting
storage techniques, and so forth.
Non-invasive prenatal blood test
A non-invasive pre-natal blood test could detect
whether a fetus will have genetic disorders such as cystic fibrosis or
sickle cell anemia, according to a study published online in the
Proceedings of the National Academy of Science, the Wall Street
Journal reports. Pre-natal diagnoses of such disorders currently are
possible through amniocentesis, a procedure that involves inserting a
needle into the uterus and carries a small risk of miscarriage,
according to the Journal.
New tests could be developed because of a
discovery by researchers at the Chinese University of Hong Kong who
found fetal DNA circulates in maternal blood, Reuters reports.
Digital blood-testing technology was used to count abnormal DNA
sequences in the pregnant woman's plasma to determine the number of
abnormal genes inherited by the fetus and the fetus' probability of
developing a genetic disorder. Also notes was that the accuracy of the
test depends on the concentration of fetal DNA in maternal blood. The
test currently is expensive and inefficient, but is offered as “proof”
that the technique can be used to diagnose fetal disorders.
Reprinted with
kind permission from
www.nationalpartnership.org. The National Partnership for Women
and Families, published by The Advisory Board Company, offers the Daily
Women's Health Policy Report as a free service that includes archives
and e-mail updates.
The future of post-natal testing and newborn screening
MLO approached Piero Rinaldo, MD, PhD,
professor of Laboratory Medicine and Pathology at Mayo Clinic in
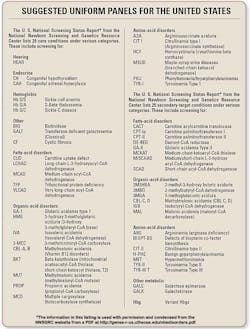
Rochester, MN, for information about newborn screening. All 50 states
screen newborns for various core conditions like hearing, congenital
hypothyroidism, sickle-cell anemia, and a host of others.
According to Dr. Rinaldo, since the 2006
recommendations of the American College of Medical Genetics (Toward a
Uniform Screening Panel and System written by Rinaldo with Michael S.
Watson, PhD; Marie Y. Mann, MD, MPH; Michele A. Lloyd-Puryear, MD, PhD; and
R. Rodney Howell, MD, editors), “the degree of implementation of a uniform
panel of 29 conditions is practically complete.”
panel, and most are also screened for the 25 secondary targets. It will
be 100% before the end of the year,” Rinaldo notes.
“In fact, more than 98% of U.S. births are screened
for that panel, and most are also screened for the 25 secondary targets. It
will be 100% before the end of the year,” Rinaldo notes.
His interests and training in pediatrics and human
genetics combine to support his ongoing studies of what he terms “the next
generation of newborn conditions, including SCID (severe combined
immunodeficiency), LSD (lysosomal storage diseases, e.g., Pompe disease and
Krabbe disease), and hyperbilirubinemia.”
Much of Dr. Rinaldo's expertise is contained in his
writings. Included among his publications are 39 items listed on PubMed, a
service of the National Library of medicine.
The listing of these articles can be found online at:
www.ncbi.nlm.nih.gov/sites/entrez?cmd=PureSearch&db=pubmed&term=%28rinaldo%20p%5BAuthor%5D%20AND%20mayo%5BAll%20Fields%5D%29.
Among them are:
- Newborn Screening of Metabolic Disorders: Recent Progress and
Future Developments, written by Piero Rinaldo with James S. Lim,
Silvia Tortorelli, Dimitar Gavrilove, and Dietrich Matern:
Nestle Nutr Workshop Ser Pediatr Program. 2008; 62:81-93;
discussion 93-96.
According to the description of this article, with
the development of tandem mass spectrometry (MS/MS), more than 40 conditions
could be detected by a single test. Thus, the evolution of newborn screening
has grown until the panel of conditions recommended by the American College
of Medical Genetics included, at the time of the article, 20 primary
conditions and 22 secondary targets, detectable by MS/MS.
- Making the case for objective performance metrics in newborn
screening by tandem mass spectrometry, written by Piero Rinaldo with
Saba Zafari, Silvia Tortorelli, and Dietrich Matern:
Ment Retard Dev Disabil Res Rev. 2006;12(4):255-261.
This article focuses on the expansion of
newborn-screening programs which might include multiplex testing by MS/MS.
The authors propose particular targets as evidence of adequate analytical
and post-analytical performance, based on their experience with performance
metrics in a program limited to MS/MS testing.
- Combined newborn screening for succinylacetone, amino acids, and
acylcarnitines in dried blood spots by Piero Rinaldo with Coleman
Turgeon, Mark J. Magera, Pierre Allard, Silvia Tortorelli, Dimitar
Gavrilow, Devin Oglesbee, Kimlo Raymond, and Dietrich Matern:
Clin Chem. 2008 Apr;54(4):657-664. Epub 2008 Feb 15.
This article focuses on tyrosinemia type 1, a
disorder that can cause early death if not treated. Because screening
newborns for TYR1's diagnostic marker is problematic, the authors developed
a new assay using dried blood spots in concert with flow-injection MS/MS as
a solution.
See page 18 for the
National Newborn Screening and Genetics Resource Center's Suggested Uniform
Panels for the United States.
NOTE: CE Test questions include all
articles of this month's Cover Story section, except the product
announcement.