To earn CEUs, see current test at www.mlo-online.com under the CE Tests tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
- Describe three of the mosquito species that transmit WNV, as well as name several virulent strains of WNV from around the world.
- Describe the process of enhanced transmission by mosquitoes of various WNV strains.
- Discuss WNV diagnostics, such as lateral flow and real-time PCR, and understand the care to be taken with molecular techniques.
- Discuss the emergence of new WNV vaccines, as well as current antiviral targets.
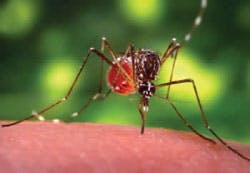
West Nile virus: the current climate
By Natalie A. Prow, PhD; Alexander A. Khromykh, PhD; and Roy A. Hall, PhD
West Nile virus (WNV) is a member of the Japanese encephalitis antigenic complex of flaviviruses, which are responsible for a range of clinical syndromes in humans, from mild fever to fatal encephalitis. WNV is transmitted by many different mosquito species including Aedes, Culex, and Anopheles. The virus is maintained in nature via a mosquito-bird-mosquito cycle, where humans and other animals are incidental hosts. Since the first detection of WNV in the Western Hemisphere in 1999, the virus has spread rapidly across North America and as far south as
Argentina.1 This review focuses on recent outbreaks of WNV in the Americas and other parts of the world, as well as the latest advances in diagnostics, vaccines, and antiviral therapies.
Human disease: the outbreak continues
The North American outbreak of WNV disease began in New York in 1999 and has continued through 2008, with 1,338 new cases reported to the Centers for Disease Control and Prevention (CDC) for the period Jan. 1, 2008, through Dec. 31, 2008. While the total number of cases is down from 2007 for the same period (3,630 cases), the proportion of WNV cases reported as West Nile meningitis or encephalitis did increase at an alarming rate. In 2007, 34% of WNV cases were reported as meningitis or encephalitis (neuroinvasive disease), whereas in 2008 this proportion rose to 50%.2 The high proportion of neuroinvasive disease among reported cases may reflect surveillance reporting bias, with severe cases more likely to be reported than mild cases. This trend, however, is not unique to the United States, with an increase in incidence of neuroinvasive WNV infections also occurring in Europe. Hungary previously reported a yearly average of six cases of WNV neuroinvasive infections between 2003 and 2007. Fourteen confirmed cases, however, were documented in 2008.3 The first two cases of human WNV neuroinvasive infections were also reported in Italy in 2008.4
Continuing outbreaks of WNV in the Americas and other regions of the world has prompted several countries to conduct seroprevalence and vector-competence studies to evaluate the risk of establishment of virulent strains of WNV into their particular region. While a naturally attenuated strain of WNV (WNVKUN) is endemic in Australia, virulent strains of WNV have not been detected in Australian mosquitoes; and locally transmitted cases of WNV fever or neuroinvasive disease have not been reported. To assess the risk of virulent strains of WNV to Australian biosecurity, the ability of Australian-mosquito species to transmit the virulent North American strain of WNV (WNVNY99) was evaluated. A number of Culex species demonstrated a high vector competence for WNVNY99, with some populations of Culex annulirostris, the primary Australian vector of WNVKUN, displaying transmission rates up to 84%.5 Therefore, Australia possesses a number of competent mosquito species that could facilitate local transmission of virulent strains of WNV, should they be introduced.
Emergence of highly virulent strains of WNV
Due to the unprecedented pattern of large annual epidemics of human neuroinvasive disease in North America, researchers have investigated changing patterns of virulence in WNV isolates. In vivo studies of a 2001 isolate from Louisiana (LSU-AR101) revealed increased neuroinvasiveness and neurovirulence in mice compared to the prototypic NY-99 strain, even at low doses. Further studies indicate that a distinct subclade of highly neurovirulent strains occur in North America; however, there is no temporal association that would suggest a recent emergence.6
Interestingly, WNV incursion into Latin America and the Caribbean Islands has resulted in surprisingly low levels of human, avian, and equine morbidity and mortality despite evidence that the WNV strains circulating in those regions are similar to those in North America.7 Host factors that may also contribute to WNV encephalitis in humans include hypertension, immunosupressing conditions, and cardiovascular disease determined by an age-, gender- and race/ethnicity-matched case-control study.8
Enhanced transmission of WNV strains by mosquitoes
In 2002, there was a dramatic and unexpected increase in the number of WNV infections in the United States from 66 infections in 2001 to 4,156 human infections in 2002. Furthermore, the 2002 transmission season lasted longer than previous years with some cases occurring in December. This was presumably due to the incursion of the virus into several of the southernmost states of the United States where weather conditions support year-round mosquito breeding. The sudden rise in WNV cases also coincided with the emergence of a new WNV genotype (WN02), that continues to be the predominate virus circulating in the Americas.9 An interesting feature of the WN02 genotype is its enhanced ability to replicate and disseminate in Culex mosquitoes. The extrinsic incubation period (time between infection of the mosquito and its ability to infect a vertebrate host) for viruses in the WN02 genotype in Culex mosquitoes, was found to be up to four days shorter than viruses from the NY99 genotype.10 This enhanced efficiency to transmit WN02 by mosquito vectors has likely contributed to the dramatic increase in WNV cases in the United States since 1999-2001. To further exacerbate the problem, irrigation has recently been linked to a greater incidence of human and veterinary WNV cases in the United States by creating appropriate habitats for mosquito breeding.11
WNV diagnostics: Are we missing cases?
The diagnosis of an acute or convalescent WNV infection can be confirmed by various serological assays such as enzyme immunoassay or EIA, immunofluorescence assay (IFA), or neutralization test or NT, which are conducted by a growing number of laboratories. In 2007, an external quality-assurance program involving 27 laboratories from 20 different countries in Europe, the Middle East, the Americas, and Africa assessed the diagnostic quality of serological detection of WNV. This program determined that a key issue facing WNV diagnostics was false positives due to cross-reaction of antibodies to closely related flaviviruses.12 Furthermore, the persistence of WNV-reactive IgM in serum for several months after infection, suggests that a serological assay that unequivocally distinguishes a recent WNV infection from past exposure to the virus, based on analysis of a single specimen, remains elusive.
Current efforts to improve WNV diagnostics have focused on the evaluation of new technologies, based on lateral flow detection of IgM13 and real-time PCR.14 The lateral flow assay was compared to commercially available tests and was found to be an effective, qualitative screening test that produces results comparable to the commercially available kits. This new assay is rapid and does not require automated equipment, tedious calculations, or special chemicals or reagents used in traditional enzyme immunoassays. Lateral flow is only suitable, however, for situations where a small number of samples is being tested.13 With the emergence of highly neuroinvasive strains of WNV, virus genotyping may have important applications, not only for diagnostic and prognostic value but also for surveillance and epidemiological studies. Zaayman and colleagues detail the development of the first rapid, sensitive real-time PCR (polymerase chain reaction) designed for the simultaneous detection and genotyping of WNV. The assay detects and differentiates WNV strains by means of dissociation-curve analysis, using fluorescence resonance energy transfer or FRET probe technology.14 This test will not only allow the investigation of strain variability in clinical specimens but also may allow differentiation between virulent and avirulent strains. Furthermore, due to the specificity of real-time PCR, false positive due to cross-reaction from heterologous flaviviruses in serological assays would no longer be a problem. Care must be taken, however, with molecular techniques consistent with our understanding of WNV viraemia and antibody production. Serum levels of WNV RNA typically peak before symptoms appear and rapidly decline over several days as antibody production begins. Therefore, using only RNA detection methods can lead to false negatives for some WNV infections.15 Testing for viral RNA coupled with detection of viral-specific IgM remains the most accurate way to diagnose new cases of WNV infection.
In the search for more discriminating diagnostic tools for WNV, Hobson-Peters, et al,16 characterized a linear glycosylated peptide in the E protein of WNV that was immunogenic during infection of horses. Of note, only serum from animals infected with the virulent WNVNY99 strain — but not with the benign WNVKUN strain — appeared to recognize the epitope. Although the sample size was small, this peptide represents a potentially strain-specific diagnostic tool for the surveillance of WNV infections in Australia and possibly other countries.
A new generation of safe WNV vaccines
The large number of cases of human disease attributed to WNV infection, coupled with the absence of effective treatments for this disease, has fueled the need for suitable vaccine candidates. A new generation of vaccines that are stable, safe, and highly potent in inducing protective immunity against WNV infection are emerging. Vaccine strategies include live attenuated, inactivated, recombinant subunit, viral-vectored, chimeric viruses, and novel nuclei- acid-based vaccine candidates.17 While there are advantages and disadvantages to all vaccine strategies, DNA (deoxyribonucleic acid) vaccines represent an attractive prospect, since plasmid DNA can be highly purified, preventing adventitious viruses from contaminating the vaccine preparation. Furthermore, DNA is stable at ambient temperatures and can be delivered in very small quantities. While research to date has found DNA vaccines to be a safe option, the long-term safety associated with DNA vaccination remains to be seen. In 2005, a recombinant plasmid that expressed WNV prM (premembrane) and E (envelope) proteins was licensed to Fort Dodge Laboratories for use in horses, becoming the world's first veterinary DNA vaccine.17
One example of a novel DNA vaccine-based approach includes an elegant vaccine based on a replicon system derived from WNVKUN.18 In this strategy, a capsid-deficient WNV genome is co-transcribed with mRNA (messenger ribonucleic acid) for the full-length capsid protein from separate promoters in a single plasmid. In transfected cells, the capsid-deleted RNA transcript is replicated and translated to produce secreted virus-like particles lacking the nucleocapsid. This RNA is also packaged with the help of co-expressed capsid protein to form secreted single-round infectious particles (SRIPs) that deliver the RNA into neighboring cells. In SRIP-infected cells, the RNA is replicated again and produces additional virus-like particles; but in the absence of mRNA for capsid translation, no SRIPs are formed and no further spread occurs. The SRIPs DNA vaccine approach was able to elicit protective virus-neutralizing antibodies in horses and protect mice from an otherwise lethal dose of WNV after a single dose. Furthermore, SRIPs DNA vaccination also elicited cytotoxic T-cell responses, thus providing additional mechanisms of protection compared to conventional non-replicating DNA and inactivated virus vaccines.18
Current antiviral targets
In the absence of a suitable commercial human vaccine, antiviral therapy could potentially reduce morbidity and mortality associated with WNV infections; however, no effective drugs are currently available. To date, research has focused on the envelope glycoprotein, the NS3 protease and helicase, and the NS5 methytransferase and RNA-dependent RNA polymerase (RdRp) as targets for antiviral therapy. Since human cells lack RNA-dependent RNA polymerases, the latter appears as a promising target for antivirals against WNV.19 With the recent resolved crystal structure of the WNV NS5 RdRp, structure-function studies are now possible, paving the way for a new generation of antiviral drugs to inhibit RdRp activity.20 Viral polymerase activity can be targeted using either nucleoside analogs or non-nucleoside compounds, the latter targeting allosteric sites in the protein.
The development of high-throughput, in vitro, cell-based screening assays will identify general inhibitors of virus growth including inhibitors of the RdRp. Active compounds can then be assessed for inhibition of RdRp activity in vivo.21
Conclusions
West Nile virus remains a major global public-health concern with the emergence of viral strains that promote transmission by mosquito vectors and strains that are associated with more severe neurological disease. The development of improved diagnostic tools, safe and effective vaccines, and the identification of novel antiviral targets present exciting avenues of research that will enable better management of human disease associated with WNV.
All three authors are based at the University of Queensland in Australia. Natalie A. Prow, PhD, is an Australian Research Council Postdoctoral Fellow; she can be reached by e-mail at [email protected] . Alexander A. Khromykh, PhD, is a professor of Virology at the School of Chemistry and Molecular Biosciences. Roy A. Hall, PhD, is reader in Molecular Virology at the School of Chemistry and Molecular Biosciences.
References
- Petersen LR. Global Epidemiology of West Nile Virus. In: West Nile Encephalitis Virus Infection: Viral pathogenesis and the Host Immune Response. Diamond MS. Springer. 2008;1-24.
- The West Nile Virus Homepage U.S. Centers for Disease Control and Prevention. Available at www.cdc.gov/WestNile . Accessed March 5, 2009.
- Krisztalovics K, Ferenczi E, Molnar Z, Csohan A, et al. West Nile virus infections in Hungary. Eurosurveillance. 2008;13(45)pii:19030.
- Grazzini G, Liumbruno GM, Pupella S, Silvestri AR, et al. West Nile virus in Italy: a further treat to blood safety, a further challenge to the blood system. Blood Transfus. 2008;6:235-237.
- Jansen CC, Webb CE, Northhill JA, Ritchie SA, et al. Vector competence of Australian mosquito species for a North American Strain of West Nile Virus. Vector borne Zoonotic Dis. 2008;8(6):805-811.
- Iyer AV, Boudreaux MJ, Wakamatsu N, Roy AF, et al. Complete genome analysis and virulence characteristics of the Louisiana West Nile virus strain LSU-AR01. Virus Genes. 2009;38:204-214.
- Petersen LR, Hayes EB. West Nile virus in the Americas. Med Clin North Am. 2008;92(6):1307-1322.
- Murray KO, Koers E, Baraniuk S, Herrington E, et al. Risk factors for Encephalitis from West Nile Virus: A Matched Case-Control Study Using Hospitalized controls. Zoonoses Public Health. 2009(Jan. 17);PMID:19175570.
- Blitvich BJ. Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev. 2008; 9(1):71-86.
- Moudy RM, Meola MA, Morin L-L, Ebel GD, et al. A newly emergent genotype of West Nile Virus is transmitted earlier and more efficiently by Culex mosquitoes. Am J Top Med Hyg. 2007;77(2):365-370.
- Gates MC, Boston RC. Irrigation linked to a greater incidence of human and veterinary West Nile virus cases in the United States from 2004 to 2006. Prev Vet Med. 2009(Jan. 29);PMID:19185941.
- Niedrig M, Mantke OD, Altmann D, Zeller H. First international diagnostic accuracy study for the serological detection of West Nile virus infection. BMC Infect Dis. 2007;7:72.
- Sambol AR, Hinrichs SH. Evaluation of a new West Nile Virus lateral-flow rapid IgM assay. J Virol Methods. 2009(Jan. 10);PMID:19138705.
- Zaayman D, Human S, Venter M. A highly sensitive method for the detection and genotyping of West Nile virus by real-time PCR. J Virol Methods. 2009(Jan. 10);PMID:19138708.
- Prince HE, Calma J, Pham T, Seaton BL. Frequency of missed cases of probable acute West Nile virus (WNV) infection when testing for WNV RNA alone or WNV Immunoglobulin M alone. Clin Vaccine Immunol. 2009(Feb. 18);PMID:19225078.
- Hobson-Peters J, Toye P, Sanchez MD, Bossart KN, et al. A glycosylated peptide in the West Nile envelope protein is immunogenic during equine infection. J Gen Virol. 2008;89:3063-3072.
- Khromykh AA, Chang DC, Hall RA. Vaccine development against West Nile Virus. In: West Nile Encephalitis Virus Infection: Viral pathogenesis and the Host Immune Response, Diamond MS, ed. Springer. 2009;428-451.
- Chang DC, Liu WJ, Anraku I, Clark DC, et al. Single-round infectious particles enhance immunogenicity of a DNA vaccine against West Nile Virus. Nat Biotechnol. 2008;26(5):571-577.
- Sampath A, Padmanabhan R. Molecular targets for Flavivirus drug discovery. Antiviral Res. 2008;81:6-15.
- Malet H, Egloff M-P, Selisko B, Butcher RE, et al. Crystal structure of the RNA polymerase domain of the West Nile virus non-structural protein 5. J Biol Chem. 2007;282(14):10678-10689.
- Malet H, Masse N, Selisko B, Romette JL, et al. The Flavivirus polymerase as a target for drug discovery. Antiviral Res. 2008;80(1): 23-35.
West Nile virus 2009: from emerging to endemic in 10 short years
By Chris Gardner, BS
The first West Nile virus (WNV) cases that made chilling headline news in 1999 brought only questions: What is causing birds and humans to become ill and die? An unknown pathogen? How is it transmitted? How deadly? Today we know that WNV is mosquito-borne, transmitted by a variety of mosquito species, and supported by numerous mammal hosts, including animals and migratory birds. WNV is also now known to be transmitted via transfusion, organs/tissue transplant, and in utero and through breastfeeding. In just 10 years, WNV has gone from “emerging” to “endemic” and joined the ranks of other arboviruses (ARthropod-BOrne viruses) that periodically plague different regions of North America, although at a much lower frequency than WNV.
Table 1: Cases of arboviral disease reported to Centers for Disease Control and Prevention
Total human WNV cases reported to the CDC: These numbers reflect both mild and severe human disease cases occurring each year as reported to ArboNET by state and local health departments. ArboNET is the national, electronic surveillance system established by CDC to assist states in tracking West Nile virus and other mosquito-borne viruses.
Click here for close up of chart
Joining the ranks of the arboviruses
West Nile virus is a flavivirus closely related to Japanese encephalitis, St. Louis encephalitis (SLE), and Dengue viruses. Not a newly discovered pathogen after all, WNV was simply new to the United States in 1999. Because of its rapid and persistent spread across the country, WNV is probably the most notorious U.S. virus since HIV and the blood-borne hepatitis viruses. The peak incidence for WNV was in 2003, with 9,862 cases reported and 264 deaths. Most summers, some half-dozen viruses, collectively known as arboviruses, infect individuals from coast to coast in the Americas. Encephalitis is the most severe form of arboviral disease and the most commonly reported. The exception is WNV, for which both mild (West Nile fever) and neuroinvasive disease (meningitis and encephalitis are reported.
Laboratory diagnosis
Fortunately, most WNV infections tend to be mild or self-limiting — the individual with a slight fever, headache, and body ache might diagnose himself as having the “flu.” Only 20% of infections are symptomatic, and less than 1% of individuals will develop neuroinvasive disease. (The mortality rate for EEE is much higher at around 30%.) Based on symptoms alone, it is rarely possible to diagnose accurately the exact cause of febrile illness. Laboratory testing is important for establishing an accurate diagnosis and providing accurate epidemiological information about which viruses are being passed in a given region. A number of tests are currently in use or available:
Serological tests:
- IgM Capture ELISA or IFA — indicates acute infection in serum or cerebrospinal fluid;
- IgG ELISA or IFA — indicates previous exposure; fourfold increase indicates recent infection;
- Plaque reduction neutralization (PRNT) — confirmatory test for WNV in that it differentiates WNV antibodies from those directed toward SLE, a closely related but less common virus; and
- IgG aviditiy test — can help distinguish between recent and past infection.
Virus-detection tests:
- PCR — detects viral RNA; useful for detecting virus in blood products, but less useful as a diagnostic test because the window for RNA detection is short (a few days) in most patients; and
- Viral culture — not used for diagnostic testing.
New information pending publication: A recent study (data presented in abstract at the 2009 West Nile National Conference. Feb. 19-20, 2009, in Savannah, GA) has shown that some acute infections may be missed by performing only IgM or PCR testing. This suggests that there is a window when it would be of value to perform both serological and molecular testing (i.e., in the transition from viremia to immune response).
Vaccine on the horizon?
West Nile vaccines for human use have been in development since 2000 and in clinical trials since 2005 under funding by the National Institute of Allergy and infectious Disease or NIAID. The first vaccine is anticipated for approval sometime in 2009. Vaccine scientists have taken advantage of the close genetic relationship between WNV and yellow fever virus — both flaviviruses — to design a vaccine that can be used to protect humans against WNV infection. While there are no human vaccines to date, vaccines for veterinary use have been available for a number of years.
On the alert: Chikungunya virus
Travelers from outside the United States can enter the country or return home with other vector-borne infections that are not currently endemic in the Americas. Whether the virus is able to spread or remains an isolated event depends on the makeup of the local mosquito population. Chikungunya virus is a mosquito-borne virus that caused an outbreak in Italy in 2007 and now has the potential to enter and spread in the United States via mosquitoes native to certain areas. The Aedes albopictus mosquito, also known as the Asian tiger mosquito, was the primary carrier of the virus in the recent outbreak in Italy and is commonly found in southern and eastern regions of the United States. Another Aedes mosquito, A aegypti, is responsible for outbreaks in Asia and Africa, and is found in the United States, primarily in southern states.
Chris Gardner, BS, is Technical Services manager at Focus Diagnostics Inc. (www.focusdx.com ) in Cypress, CA, which currently has available commercial PCR and serological testing for Chikungunya virus.
References
- Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile Encephalitis, New York, 1999: Results of a household-based seroepidemiological survey. Lancet. 2001;358:261-264.
- General reference: CDC Division of Vector-Borne Diseases Available at http://www.cdc.gov/ncidod/dvbid/Arbor/arbdet.htm . Accessed March 3, 2009.
Available at http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount08_detailed.htm . Accessed March 3, 2009.
Available at http://www.cdc.gov/ncidod/dvbid/westnile/resources/wnv-guidelines-aug-2003.pdf . Accessed March 3, 2009. - Vaccines: Available at http://www3.niaid.nih.gov/topics/westNile/research/prevention.htm . Accessed March 3, 2009. Available at http://www.nih.gov/news/pr/apr2005/niaid-18.htm . Accessed March 3, 2009.
- Hogrefe WR, R Moore, M Lape-Nixon, M Wagner, HE Prince. Performance of Immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays using a West Nile virus recombinant antigen (preM/E) for detection of West Nile virus- and other flavivirus-specific antibodies. J Clin Microbiol. 2004;42:4641-4648.
- Available at http://www.cdc.gov/ncidod/dvbid/westnile/conf/February_2009.htm . Accessed March 3, 2009.