Human papillomavirus
(HPV) infection is one of the most common sexually transmitted
infections worldwide, affecting up to 80% of women by the age of 50.1
Increasing evidence shows that men have a similar risk of HPV infection.2
There are over 100 different types of HPV; about 40 of these affect the
anogenital area. Certain types such as HPV type 16 and type 18 cause the
majority of cervical cancers worldwide. These and other “high-risk” or
oncogenic types act in contrast to the “low-risk” or non-oncogenic HPV
types such as HPV types 6 and 11, which cause the majority of
non-cancerous lesions such as anogenital warts. HPV-associated
malignancies include most cervical cancers, the most common cancer in
women in many developing countries, as well as some vulvar and vaginal
cancers.3 HPV is also an important cause of other anogenital
cancers including most anal and a proportion of head and neck cancers in
both men and women.4,5
Screening strategies have traditionally relied on
the detection and treatment of pre-cancer lesions — not infection. In
general, in communities able to implement systematic cervical
Papanicolaou (Pap) screening, this has been a very successful approach.
Combining data from national programs in eight countries, the
International Agency for Research on Cancer (IARC) described a 90%
reduction in cervical-cancer incidence if periodic screening of the
entire adult female population is undertaken.6 In most of the
developing world, however, the cost of cervical-cancer screening
infrastructure is prohibitive. Recent research has focused on the
utility of alternative screening approaches in resource-poor
environments including the use of rapid HPV testing to triage high-risk
women.7 Molecular identification of HPV has been used to
increase the interval of Pap screening in the United States or to triage
women with atypical squamous cells of undetermined significance (ASCUS)
on Pap testing to immediate colposcopy or to repeat Pap at a one year’s
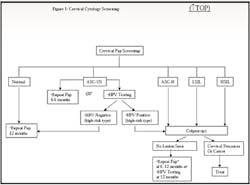
interval instead of at three to six months (see Figure 1 online).8
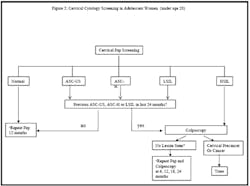
Note that recent guidelines for Pap screening in adolescents now
de-emphasize HPV testing (see Figure 2 online), given the
high-prevalence of high-risk HPV types and observation that most of
these infections are transient.9,10
The development and licensure of the HPV bivalent
(types 16, 18)11 [not FDA cleared in the United States
currently] and quadrivalent (types 6, 11, 16, 18) prophylactic vaccines12
has led to further discussions regarding the clinical use of
type-specific HPV-testing to identify already HPV-exposed women who may
benefit from vaccine.13 Currently, the Centers for Disease
Control and Prevention (CDC)/Advisory Committee on Immunization
Practices (ACIP) and the American College of Obstetricians and
Gynecologists (ACOG) guidelines do not recommend routine HPV
screening prior to the administration of the vaccine for females aged 9
to 26 years old.14 Adjunctive molecular tests are
increasingly being used to risk stratify women with abnormal biopsies.
We will review some testing methodologies
currently used in the field and discuss the potential place in screening
algorithms for new and existing HPV-diagnostic tests. Although there has
been much headway in developing screening and treatment guidelines for
other HPV-associated malignancies, such as anal cancer,15,16
this article will focus on cervical cancer.
Diagnostic tests for HPV disease
Traditionally, these tests have been the focus of
cervical-cancer screening programs in the developed world. These
programs have been successful largely because cervical cancer has such a
long pre-cancer state (typically, more than 10 years) and these
pre-cancer states are treatable. The cornerstone of most cervical-cancer
screening programs is the Pap test.17 The Pap test can be
done in two ways; both methods use cells sampled from the cervix and the
vagina using a brush or spatula. The conventional method is the Pap
smear introduced in the United States in 1941. The sensitivity of the
Pap test to detect high-grade cervical intraepithelial neoplasia (?CIN2)
ranges from 44% to 99% and specificity from 91% to 98%.18
Providers place cells on a glass slide and chemically fix them in the
office. In the newer thin-layer liquid preparations (e.g., ThinPrep;
CYTYC, Boxborough, MA), providers suspend cells in liquid transport
media, which is subsequently spun down and filtered in the laboratory
and then placed on slides for the pathologist to review. Both methods
have similar test characteristics.19 If an abnormal result is
obtained on cytology (see Figure 1 online), colposcopy, together with
topical chemicals, such as acetic acid and Lugol’s iodine, is used to
identify lesions that might have contributed to the abnormal cells seen
on cytology. Biopsies of these lesions permit the pathologist to confirm
the cytologic diagnosis and set the stage for the treatment modality
based on the findings. In resource-poor countries, visual inspection
using acetic acid (in lieu of Pap testing) can be used by trained nurses
to triage patients to colposcopy and biopsies as needed.20
Diagnostic tests for HPV infection
Incorporation of testing for HPV infection in
cervical-cancer screening programs has several potential roles both in
the developed world and in resource-poor countries. As a primary
modality, HPV testing is generally thought to be more sensitive than
cytology for detecting cervical intraepithelial neoplasia. In one
Canadian study of more than 10,000 women aged 30 to 69 years old, HPV
testing as a primary-screening modality was compared to conventional
cervical Pap testing. The sensitivity to detect biopsy proven ?CIN2
lesions using HPV testing was higher compared with the sensitivity of
Pap testing (95% vs. 55%). The corresponding specificity was similar
(94% vs. 97%).21 These results support an increased future
reliance on molecular HPV testing over traditional Pap testing,
particularly in resource-poor environments where populations are
infrequently screened. Currently, HPV testing in the developed world is
incorporated in two ways in existing screening programs. The first is
the use of HPV testing to triage women with ASCUS to immediate
colposcopy if positive for high-risk HPV infection (see Figure 1
online); if negative for a high-risk HPV type, repeat Pap testing can be
performed in one year instead of three to six months.8 The
second scenario where HPV testing is recommended is in low-risk women
over 30 years old. If found to have a negative Pap test and negative for
a high-risk HPV type, then the interval can be increased from annual
screening to every three years.18
Three principal methods exist in the laboratory
to detect HPV: 1) direct probe methods (e.g., Southern transfer
hybridization and in situ hybridization [ISH]); 2) signal
amplification (e.g., hybrid capture second-generation [HC2] assay
[QIAGEN, Gaithersburg, MD, USA]); and 3) target amplification
(polymerase chain reaction [PCR] variants). Newer test development has
focused on signal and target amplification. Of these methods, only HC2
is U.S. FDA cleared for use in HPV testing, and in the cervix only. HC2
is also approved for use in Europe (Conformit’e Europ’eenne (CE) marked).
Signal amplification
Hybrid Capture HPV DNA assay (Digene,
Gaithersburg, MD): The HC2 assay uses RNA probes specific for the
identification of certain high-risk (16, 18, 31, 33, 35, 39, 45, 51, 52,
56, 58, 59, 68) or low-risk HPV types (6, 11, 42, 43, 44). These long
RNA probes are separated into two testing cocktails based on whether
high-risk or low-risk types are to be identified. First, the patient’s
specimen (as whole HPV DNA) is separately hybridized to each of the two
testing cocktails. In each case, specific HPV DNA-RNA hybrids are
formed. This is then added to a microtiter plate coated with antibodies
specific to RNA-DNA hybrids so that the HPV DNA-RNA hybrids previously
formed can be “captured” or immobilized on the plate. Immobilized
hybrids are then bound to antibodies conjugated to alkaline phosphatase.
Excess antibodies and non-hybridized probes are removed, and a
chemiluminescent substrate is added. A luminometer is then used to
detect the remaining immobilized hybrids. A semiquantitative measure of
the viral load can be obtained based on the intensity of the light
emitted by the sample divided by the light emitted by a positive control
(expressed as relative light units) since this is proportional to the
quantity of target DNA in the patient’s specimen. To reduce cost and
time, often only high-risk probes are used in clinical evaluation and a
result of positive or negative (but not the specific HPV type) for a
high-risk group is provided.
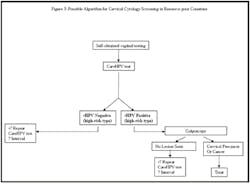
CareHPV (QIAGEN, Gaithersburg, MD):
This is a new test specifically developed for use in the developing
world and broadly based on the HC2 assay. It is meant to be rapid
(results in less than 2.5 hours in contrast to six hours with HC2),
requires minimal infrastructure, and is anticipated to be inexpensive.
The features that facilitate these differences include unique reagents
in the patient-collection device that contains non-toxic surfactants
that can quickly and directly solubilize cervical specimens without a
requirement for prolonged mechanical agitation. In addition, microtiter
plates in HC2 are replaced by magnetic beads, and temperature
requirements are altered in some steps compared to HC2. In the first
comprehensive study evaluating careHPV, more than 2,500 women in Shanxi
province in rural China were evaluated with the screening tests careHPV,
HC2, and simple visual inspection with acetic acid (VIA) using a gold
standard of colposcopy with biopsy. The sensitivity and specificity to
detect high-grade CIN (CIN 2 and higher) in cervical specimens was 90%
and 84% for careHPV, 97% and 86% for HC2, and 41% and 95% for VIA.7
This test has enormous potential for use in the developing world as it
is specifically designed to be rapidly processed by inexperienced
personnel under constraints of space and temperature while women wait
for results (see Figure 2 online).
Target amplification
There are two principal approaches used in the
detection of HPV by polymerase chain reaction (PCR): consensus PCR and
type-specific PCR. Consensus primers such as PGMY09/11 can generate a
variety of primers that can amplify and identify a wide variety of HPV
types typically in one reaction. Examples of other consensus primers
used are GP5+/6+ and short PCR fragment (SPF). The result is whether
there is HPV present or not, but not the specific HPV type. In contrast,
type-specific PCR tests target-specific sequences of viral genes, which
result in the amplification of a single HPV genotype. Therefore,
multiple PCR reactions (one per type evaluated) must be carried out on a
single specimen to determine which HPV type is present. Of these
type-specific HPV tests, only the Roche Linear Array HPV genotype test
has been developed for commercial use.
Line blot (Roche Molecular Systems,
Alameda, CA):
This is an L1 consensus primer-based PCR assay using PGMY09/11
followed by a line blot assay. In the line blot assay, multiple probes
are fixed as lines on a membrane strip. Reverse line blot hybridization
detects 27 HPV genotypes (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45,
51 to 59, 66, 68, 73, 82, 83, 84). An expanded version of the test
including detection of an additional 11 non-oncogenic types has also
been used (additionally types 61, 62, 64, 67, 69 to 72, 81, 82, 89).
This test is primarily used in the research setting and has been used in
multiple previous research studies examining the molecular epidemiology
of HPV.
Linear array (Roche Molecular Systems,
Alameda, CA):
The Linear Array HPV Genotyping Test is a commercial version of
the line blot assay that has been submitted for review but not as yet
U.S. FDA cleared. It detects 37 of the 38 types included in the line
blot assay (except the non-oncogenic HPV type 57). One study compared
the linear array to the HC2 test in 3,488 women with ASCUS on Pap
testing at baseline. The sensitivity (93% vs. 93%), specificity (48% vs.
51%), negative predictive value (99% vs. 99%), and positive predictive
value (15% vs. 15%) of the baseline detection of high-risk HPV types
(HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52) to predict biopsy-proven
cervical intraepithelial neoplasia (CIN) grade 3 at two years were
similar when comparing linear array and HC2 testing, respectively.22
INNO-LiPA HPV test (Innogenetics, Gent,
Belgium):
The INNO-LiPA HPV test is a reverse hybridization line probe assay (LiPA)
which is approved for use in Europe (Conformit’e Europ’eenne (CE) marked)
but not U.S. FDA-cleared for specific HPV typing of clinical specimens.
The LiPA test permits the use of amplimers generated by SPF as well as
MY 09/11 primers. The test uses specific probes for HPV genotypes
6, 11, 16, 18, 31, 33 to 35, 39, 40, 42 to 45, 51 to 54, 56, 58, 59, 66,
68, 70, 74. Like the line blot and linear array assays, the probes are
immobilized as parallel lines on membrane strips.
AMPLICOR (Roche Molecular Systems, Alameda,
CA):
The Roche AMPLICOR HPV test amplifies target DNA by PCR followed by
nucleic-acid hybridization to various HPV types. AMPLICOR detects the
same 13 high-risk HPV types detected by HC2 and also uses amplification
of the ?-globin gene as an internal measure of sample integrity and
adequacy. The performance characteristics to determine the presence or
absence of any high-risk HPV type (but not the specific HPV genotype) is
similar to that of the HC2 test.23 AMPLICOR, approved in
Europe, is not currently U.S. FDA-cleared for clinical use.
Other selected diagnostic tests
P16-INK4A is a cyclin-dependent
kinase inhibitor, which is overexpressed in cell lines where the
HPV-induced E7 oncogenic protein product has inactivated the
retinoblastoma protein RB. The RB protein normally arrests
growth and induces cell apoptosis in response to DNA damage. If
RB is inactivated, then unregulated cell growth may lead to
malignant transformation. P16-INK4A can be identified by
immunohistochemistry (CINtech P16-INK4A cytology kit [Dako A S,
Glostrop, Denmark]) or enzyme-linked immunosorbent assay, making
it a potential marker for risk stratification of HPV-positive
women. In a study of 24,661 women in Italy, the sensitivity and
specificity of P16-INK4A to detect CIN2 or greater was 88% and
61%, respectively. Using a strategy of HPV testing and P16-INK4A
triage compared to cervical Pap tests in the 25- to 60-year-old
age group, the relative sensitivity was 1.53. There was also no
large increase in referrals to colposcopy.24
HPV viral load in a cervical sample (the amount of HPV
DNA) can be determined by real-time PCR methods. Studies have
been inconsistent in showing a prospective relationship between
HPV viral load and the subsequent development of high-grade CIN.25
For now, this is not a standard test that is used or recommended
to risk stratify women with HPV-associated disease. HPV
DNA sequencing is also a potential method to identify
specific HPV types. Its use is limited by the inability to
sequence multiple HPV genotypes.26 Finally, because
of poor standardization and reproducibility, HPV serology as a
marker of past and/or cumulative exposure to HPV-associated
disease is not widely used outside of clinical trials.
HPV is a common sexually transmitted infection
that causes a large burden of disease including various anogenital
cancers and external genital warts worldwide. In industrialized
countries, screening for pre-cancer lesions using Pap tests has resulted
in a substantial decrease in cervical-cancer incidence. The development
of molecular-based diagnostics such as HC2 has led to screening
strategies that incorporate cytology and HPV testing to risk-stratify
women to less or more frequent screening. New evidence also suggests
that HPV testing may be a viable alternative to traditional cytologic
screening. The decrease in cervical cancer incidence in the West is in
contrast to many developing countries where cervical cancer is one of
the top two causes of cancer-related deaths in women. This difference in
cervical-cancer incidence is largely thought to be due to the absence of
cervical-cancer screening programs in many developing countries. HPV
testing may have enormous impact in the developing world as a means to
rapidly, more economically, and more easily risk stratify women who need
further evaluation and treatment. The development and incorporation into
screening of type-specific HPV testing as well as other molecular
methods (i.e., P16-INK4A might further help to more specifically
identify the smaller group of women who need intervention. Ultimately,
the widespread uptake of currently available and next-generation HPV
prophylactic vaccines will be the most effective measure to reduce the
HPV-associated disease burden worldwide.
Peter V. Chin-Hong, MD, works
in the Department of Medicine, University of California-San Francisco,
CA, as does Jeffrey D. Klausner, MD, MPH, who
is also director of STD Prevention and Control Services for the San
Francisco Department of Public Health.
References
1. Workowski KA, Berman SM. Sexually transmitted
diseases treatment guidelines, 2006. MMWR Recomm Rep.
2006;55:1-94.
2. Nielson CM, Harris RB, Dunne EF, et al. Risk
factors for anogenital human papillomavirus infection in men. J
Infect Dis. 2007;196:1137-1145.
3. Franceschi S. The IARC commitment to cancer
prevention: the example of papillomavirus and cervical cancer.
Recent Results Cancer Res. 2005;166:277-297.
4. Chin-Hong PV, Palefsky JM. Natural history
and clinical management of anal human papillomavirus disease in men
and women infected with human immunodeficiency virus. Clin Infect
Dis. 2002;35:1127-1134.
5. D’Souza G, Kreimer AR, Viscidi R, et al.
Case-control study of human papillomavirus and oropharyngeal cancer.
N Engl J Med. 2007;356:1944-1956.
6. Screening for squamous cervical cancer:
duration of low risk after negative results of cervical cytology and
its implication for screening policies. IARC Working Group on
evaluation of cervical cancer screening programmes. Br Med J (Clin
Res Ed).
1986;293:659-664.
7. Qiao YL, Sellors JW, Eder PS, et al. A new
HPV-DNA test for cervical-cancer screening in developing regions: a
cross-sectional study of clinical accuracy in rural China. Lancet
Oncol. 2008.
8. Results of a randomized trial on the
management of cytology interpretations of atypical squamous cells of
undetermined significance. Am J Obstet Gynecol.
2003;188:1383-1392.
9. Moscicki AB, Shiboski S, Hills NK, et al.
Regression of low-grade squamous intra-epithelial lesions in young
women. Lancet. 2004;364:1678-1683.
10. Wright TC, Jr., Massad LS, Dunton CJ, Spitzer
M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the
management of women with abnormal cervical cancer screening tests.
Am J Obstet Gynecol. 2007;197:346-355.
11. Harper DM, Franco EL, Wheeler C, et al.
Efficacy of a bivalent L1 virus-like particle vaccine in prevention
of infection with human papillomavirus types 16 and 18 in young
women: a randomised controlled trial. Lancet.
2004;364:1757-1765.
12. Ault KA. Effect of prophylactic human
papillomavirus L1 virus-like-particle vaccine on risk of cervical
intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in
situ: a combined analysis of four randomised clinical trials.
Lancet. 2007;369:1861-1868.
13. Wright TC, Jr., Bosch FX. Is viral status
needed before vaccination? Vaccine. 2008;26 Suppl 1:A12-15.
14. Markowitz LE, Dunne EF, Saraiya M, Lawson HW,
Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine:
Recommendations of the Advisory Committee on Immunization Practices
(ACIP). MMWR Recomm Rep. 2007;56:1-24.
15. Chin-Hong PV, Berry JM, Cheng SC, et al.
Comparison of patient- and clinician-collected anal cytology samples
to screen for human papillomavirus-associated anal intraepithelial
neoplasia in men who have sex with men. Ann Intern Med.
2008;149:300-306.
16. Goldie SJ, Kuntz KM, Weinstein MC, et al. The
Clinical Effectiveness and Cost-effectiveness of Screening for Anal
Squamous Intraepithelial Lesions in Homosexual and Bisexual
HIV-Positive Men. JAMA. 1999;281:1822-1829.
17. Vilos GA. The history of the Papanicolaou
smear and the odyssey of George and Andromache Papanicolaou.
Obstet Gynecol. 1998;91:479-483.
18. Wright TC, Jr., Cox JT, Massad LS, Twiggs LB,
Wilkinson EJ. 2001 Consensus Guidelines for the management of women
with cervical cytological abnormalities. JAMA.
2002;287:2120-2129.
19. Ronco G, Cuzick J, Pierotti P, et al.
Accuracy
of liquid based versus conventional cytology: overall results of new
technologies for cervical cancer screening: randomised controlled
trial. BMJ. 2007;335:428.
20. Sankaranarayanan R, Basu P, Wesley RS, et al.
Accuracy of visual screening for cervical neoplasia: Results from an
IARC multicentre study in India and Africa. Int J Cancer.
2004;110:907-913.
21. Mayrand MH, Duarte-Franco E, Rodrigues I, et
al. Human papillomavirus DNA versus Papanicolaou screening tests for
cervical cancer. N Engl J Med. 2007;357:1579-1588.
22. Gravitt PE, Schiffman M, Solomon D, Wheeler
CM, Castle PE. A comparison of linear array and hybrid capture 2 for
detection of carcinogenic human papillomavirus and cervical
precancer in ASCUS-LSIL triage study. Cancer Epidemiol Biomarkers
Prev. 2008;17:1248-1254.
23. Carozzi F, Bisanzi S, Sani C, et al.
Agreement between the AMPLICOR Human Papillomavirus Test and the
Hybrid Capture 2 assay in detection of high-risk human
papillomavirus and diagnosis of biopsy-confirmed high-grade cervical
disease. J Clin Microbiol. 2007;45:364-369.
24. Carozzi F, Confortini M, Palma PD, et al. Use
of p16-INK4A overexpression to increase the specificity of human
papillomavirus testing: a nested substudy of the NTCC randomised
controlled trial. Lancet Oncol. 2008.
25. Sun CA, Lai HC, Chang CC, Neih S, Yu CP, Chu
TY. The significance of human papillomavirus viral load in
prediction of histologic severity and size of squamous
intraepithelial lesions of uterine cervix. Gynecol Oncol.
2001;83:95-99.
26. Boulet GA, Horvath CA, Berghmans S, Bogers J.
Human papillomavirus in cervical cancer screening: important role as
biomarker. Cancer Epidemiol Biomarkers Prev. 2008;17:810-817.
Figure 1. Cervical cytology screeningKey: ASCUS: atypical squamous cells of undetermined significance ASC-H: atypical squamous cells: cannot exclude high-grade SIL LSIL: low-grade squamous intraepithelial lesions HSIL: high-grade squamous intraepithelial lesions *If HSIL and no lesion seen on colposcopy, most guidelines recommend diagnostic excisional procedure.
2. Cervical cytology screening in adolescent women (under age 20)
Key: ASCUS: atypical squamous cells of undetermined significance ASC-H: atypical squamous cells: cannot exclude high-grade SIL LSIL: low-grade squamous intraepithelial lesions HSIL: high-grade squamous intraepithelial lesions *If HSIL and no lesion seen on colposcopy, most guidelines recommend diagnostic excisional procedure.
resource-poor countries