Hydrogen sulfide heightens disease in TB, suggests a new therapeutic target
A new culprit—hydrogen sulfide—has been found for the deadly infectious disease tuberculosis (TB). Hydrogen sulfide gas is known for its rotten egg smell, yet it has normal physiological roles in the human body to communicate among cells.
When tuberculosis bacteria invade the lung, however, the amounts of hydrogen sulfide in the lung microenvironment appear to greatly increase, and this makes the microbe more virulent and better able to block the body's protective immune response, according to research led by Andries "Adrie" Steyn, PhD, a University of Alabama at Birmingham professor of microbiology.
The source of this hydrogen sulfide? Mycobacterium tuberculosis bacteria, or Mtb, are able to induce human macrophage immune cells to produce more hydrogen sulfide. Thus, Mtb exploits macrophage metabolism to increase Mtb virulence. During the disease, Mtb grows and safely persists inside macrophages, the immune cells that normally should have protected the lungs by killing the engulfed bacteria. In a bacterial sense, the Mtb become wolves in sheep's clothing.
Two research papers by Steyn and colleagues—the first descriptions of this unexpected role for host-generated hydrogen sulfide in bacterial pathogenesis—are published in Nature Communications and Proceedings of the National Academy of Sciences (PNAS).
"Tuberculosis is responsible for about a million and a half deaths each year, and roughly 2 billion people are latently infected with Mtb worldwide," Steyn said. "Our results indicate that Mtb exploits host-derived hydrogen sulfide to promote growth and disease and suggest that host-directed therapies targeting hydrogen sulfide production may be useful for the management of tuberculosis and other microbial infections."
Hydrogen sulfide has roles in normal physiology as one of three gasses—along with nitric oxide and carbon monoxide—that can diffuse from producer cells to send a signal to nearby cells. However, when Mtb invades macrophage cells, Steyn and colleagues found a 34-fold increase in the level of the macrophage enzyme cystathionine beta-synthase, or CBS, a major producer of hydrogen sulfide in mammalian cells.
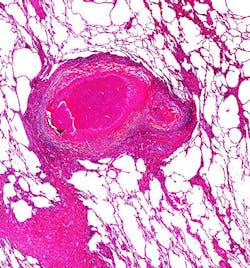
So, in the Nature Communications paper, the researchers examined the roles of CBS and different levels of hydrogen sulfide using a variety of approaches. The human tuberculosis lung tissue experiments, described in the PNAS paper, showed that hydrogen sulfide is produced at a spectrum of tuberculosis lesions that are the sites of Mtb infection.
Steyn and colleagues note that their findings highlight the hydrogen sulfide-producing enzyme CSE as a potential therapeutic target to restrain tuberculosis disease; one current drug that is used to treat rheumatoid arthritis, D-penicillamine, targets CSE.