HIV Ag-Ab assay
BioRad’s BioPlex 2200 HIV Ag-Ab assay uses its multiplex technology to simultaneously detect and differentiate HIV Ag-Ab overall result, with a) HIV-1 p24 Ag; b) HIV-1 Ab (Groups M & O); and c) HIV-2 Ab. Thanks to its sensitivity to the HIV-1 p24 antigen and the ability to differentiate between HIV-1 and HIV-2 antibodies, the BioPlex 2200 HIV Ag-Ab assay detects early acute infections and guides choice of supplemental testing through the CDC algorithm. Early detection enables early treatment, saving and improving patients’ lives.Bio-Rad Laboratories
Clostridium difficile test
Roche’s cobas Cdiff Test is an automated, qualitative in vitro diagnostic test that utilizes real-time polymerase chain reaction (PCR) technology. It is intended for use as an aid in the diagnosis of Clostridium difficile infection in humans in conjunction with clinical and epidemiological risk factors. The test runs on the automated cobas 4800 system.Roche Diagnsotics
HIV 1/2 assay
Chembio Diagnostic Systems, Inc., is now marketing and selling its SURE CHECK HIV 1/2 Assay, which was previously marketed in the United States by Alere, Inc., as Clearview COMPLETE HIV 1/2 Assay, Part #92111. This change is in name only; the product has not undergone any changes in format or formulation and does not require re-validation. This product continues to be manufactured exclusively at Chembio’s FDA-registered, cGMP-compliant and ISO 13485-certified facility in Medford, NY. SURE CHECK is an easy-to-use, self-contained collection and test device and is FDA-approved/CLIA waived (for fingerstick and whole blood). It requires only 2.5 µL of fingerstick or venous whole blood, serum, or plasma.Chembio Diagnostic Systems, Inc.
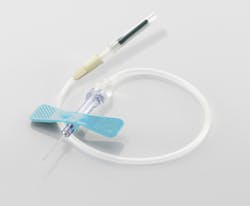
Safety blood collection set
The VACUETTE Safety Blood Collection Set from Greiner Bio-One North America, Inc., offers end users an opportunity to decrease the risk of needlestick injuries through an intuitive, in-vein activated safety feature. In addition to the increased level of safety for the end-user and the patient, this Safety Blood Collection Set also has a visual “flash” that clearly shows when the phlebotomist has entered the vein.Greiner Bio-One
Immunofluorescence antibody assays
MBL International Corporation has been manufacturing quality immunofluorescence antibody assays (IFA) infectious disease kits and components for over 30 years, offering test systems and antigen substrate slides for Herpes Simplex 1 and 2, Measles, Epstein-Barr Virus, CMV, Borrelia burgdorferi, Chlamydia, Influenza A and B, and many more.MBL International
Simplified molecular testing platform
Solana, a simplified molecular testing platform, is making molecular diagnostics faster and easier without sacrificing performance. Solana combines Quidel’s proprietary helicase-dependent amplification (HDA) with fluorescence detection, in an actionable timeframe. Culture confirmation is not required. Get confirmatory Group A Strep (GAS)results in approximately 30 minutes. Solana GAS can also be used to confirm rapid Strep A test results. Test a single specimen or batch up to 12 at once, allowing for prompt patient management even when volumes are higher.Quidel Corporation
STI multiplex array
Randox Biosciences’ newly upgraded Sexually Transmitted Infection (STI) Multiplex Array simultaneously detects 10 bacterial, viral, and protozoan infections including primary, secondary, and asymptomatic co-infections for a complete infection profile in one simple test. The assay is based on a combination of multiplex PCR and biochip array hybridization. Innovative PCR priming technology permits high discrimination between multiple targets. Analysis can be completed from template DNA through PCR to data readout in less than six hours. The array is CE marked for routine clinical use.Randox Biosciences
Multiplex MDx system
ePlex is a sample-to-answer multiplex molecular diagnostics system which integrates sample preparation steps including extraction and amplification, together with the company’s proprietary eSensor detection technology, to allow the detection of multiple molecular targets on a single test cartridge. The ePlex Respiratory Pathogen Panel (RP) is the first of many assays expected to be available on the ePlex System. With less than two minutes of operators’ hands-on time, the ePlex RP Panel detects 20 viral and three bacterial targets in nasopharyngeal specimens.GenMark Diagnostics, Inc.
Trichomonas vaginalis assay
The Aptima Trichomonas vaginalis assay is a nucleic acid amplification test (NAAT) for the diagnosis of trichomoniasis. The CDC’s 2015 STD Treatment Guidelines recommend the use of highly sensitive and specific tests for detecting T. vaginalis. NAAT-based technology is highly sensitive, often detecting three to five times more T. vaginalis infections than wet-mount microscopy. The Aptima Trichomonas vaginalis assay overcomes the challenges associated with traditional, less sensitive methodologies and makes it a reliable test to diagnose T. vaginalis infections. It is estimated that 3.7 million people in the United States are currently infected with trichomoniasis with a worldwide incidence of 276.4 million new cases each year, making trichomoniasis more prevalent than chlamydia and gonorrhea combined. Multiple sample types make it easy to use the Aptima Trichomonas vaginalis assay, orderable either as a standalone test or in addition to the Aptima Combo 2 assay for CT/NG testing: Vaginal Swab (Clinician collected), Unisex Swab (Female endocervical/male urethral), Female urine (Female urine sample type is FDA cleared for the Tigris system only) , and the ThinPrep Pap Test Vial. The Aptima Trichomonas vaginalis assay is FDA-cleared for in vitro diagnostic use.Aptima
About the Author
Sign up for our eNewsletters
Get the latest news and updates