The prevalence, pathogenesis and diagnostics of celiac disease
While biopsies for celiac disease (CD) remain the standard screening practice in the United States, European gastroenterologists and laboratories are steadily moving away from this invasive procedure, except to clarify particularly difficult cases. One of the key reasons for this change in diagnostic approach is the multitude of different serological assay methods now available to labs. Many of these blood-based assays have been developed with greater specificity and sensitivity than ever before, enabling standard diagnostic criteria to be based on detecting CD-specific antibodies. Specifically, those against tissue transglutaminase (tTG-IgA) and deamidated (modified) gliadin peptides (DGP), immunoglobulin (Ig)A and IgG.
Celiac disease
CD is a lifelong, gluten-sensitive autoimmune disease deriving from environmental (gluten) and genetic factors (human leukocyte antigen [HLA] and non-HLA genes). CD is becoming more prevalent and common in developed regions of the world, such as the U.S. and Europe, as increased awareness and detection of the disease continues to grow. The disease presents with gastrointestinal symptoms, non-gastrointestinal symptoms or no symptoms at all. It usually manifests with severe gastrointestinal problems such as diarrhea, vomiting, abdominal pains and cramps. However, about half of CD patients present with non-gastrointestinal symptoms, which can include anemia, osteoporosis, skin conditions and weight loss.
CD was originally thought to affect European populations exclusively but is currently prevalent around the world. Areas such as South America, Asia, the Middle East and Africa, previously thought to be unaffected by CD, are now believed to have been underdiagnosed, contributing to the notion that CD is becoming one of the most common autoimmune and genetic diseases.
It is thought that CD generally followed humans’ evolutionary migratory and dietary flows—from once feeding primarily on meat, fruits and vegetables, to the development and prominence of farming, when gluten-containing cereals such as wheat, barley and rye became an integral part of a diet. As human expansion continued, farming became more of a staple way of life and agricultural practices, as well as the gradual replacement of indigenous inhabitants, introduced genetic anomalies into the population leading to increased gluten sensitivity.
Disease pathogenesis
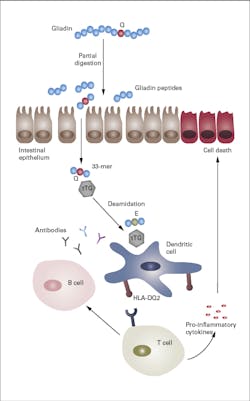
CD is typically activated by a combination of environmental and genetic causes. The main genetic factor derives from the HLA-DQ genes, DQ2 and DQ8. In genetically pre-disposed individuals, disease of the intestinal tract (enteropathy) is triggered by the immune system’s over-response to the prolamins within gluten, particularly gliadin. Gliadin peptides are not fully digested in the small intestine, enabling the gliadin remnants to be taken up into the intestinal wall and the surrounding intestinal connective tissue. At this point, the enzyme tTG deamidates (modifies) the gliadin by converting the amino acid glutamine into glutamate. The DGP affect intestinal permeability causing an immune reaction in CD patients because of their resistance to gastrointestinal enzymes. This process results in the production of antibodies against DGP and the bodies’ own tTG, and the secretion of inflammatory cytokines. The inflammation of the small-intestinal epithelium leads to atrophy of the intestinal villi. This cascade results in swelling of the small intestine and the variety of gastrointestinal symptoms mentioned above.
CD-specific antibodies and diagnosis
The prolamin trigger
Gluten is a protein found in cereals such as wheat, barley, rye and oat, amalgamating the proteins prolamin and glutelin. Most of the proteins in food that are responsible for immune reactions in CD are the prolamins. Because of their high glutamine content and certain specific sequence patterns, prolamins are resistant to gastrointestinal enzymes that protect the small intestine from autoimmune response. The incomplete gastrointestinal digestion of the gluten leads to the presence of gluten-derived gliadin peptides. The ingestion of prolamins from cereals causes changes in the small intestine mucous membrane of celiac patients leading to a syndrome of poor absorption, causing an autoimmune response after the ingestion of gluten. When screening for CD, it is mandatory that the individuals are on a regular gluten-containing diet as the CD-specific antibodies disappear when on a gluten-free diet.
New ESPGHAN diagnostic guidelines
HLA testing and presence of symptoms are not compulsory criteria for a serology-based diagnosis. Presence of HLA-DQ2 and/or DQ8 does not constitute a diagnosis of CD, nor does it mean one will ever develop it. However, carrying HLA-DQ2/DQ8 increases the risk of developing CD from the overall average of one to three percent of the population.
The current diagnostic landscape
The current environment of laboratory testing can be quite challenging for laboratories in the diagnosis of CD simply because of the recent burgeoning and availability of a variety of methods and approaches. Very different testing systems now exist with diverse diagnostic algorithms to test for the disease, so it becomes increasingly difficult for labs to decide how to implement various modalities into their routines.
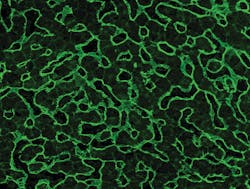
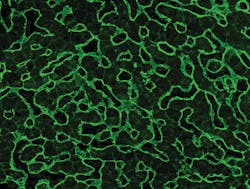
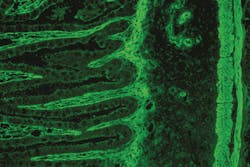
For example, the highly specific indirect immunofluorescence test (IIFT) allows for the use of a variety of different tissues, including small intestine and liver. Different tissues may require a different set of experiences and skills in reading the fluorescent patterns from the tissues. In monospecific assays, such as ELISA and immunoblot, there are different parameters including the anti-tTG and anti-DGP testing, as well as different combinations of these parameters. Assays that specifically test for antibodies against deamidated gliadins also represent a major advance in diagnostic testing. These tests are more sensitive and accurate in determining CD in that deamidated gliadin antibodies are more likely found in CD patients than native gliadin antibodies.
One of the biggest challenges gastroenterologists and laboratories still face, however, is diagnosing patients who exhibit all the symptoms of CD yet have no antibodies. At this time, there are no real criteria that would help physicians in diagnosing these types of patients. In response, researchers are trying to determine a biomarker, but as of yet none have been found. This situation remains an obstacle.
It is expected that the ESPGHAN guidelines will become more widespread outside of Europe, resulting in fewer biopsies over time—making it easier for both patients and gastroenterologists. With screening of serological markers becoming more sensitive and accurate, these guidelines would inevitably be the standard of diagnosis for the detection of CD, globally.
About the Author

Yara Burmeister
serves as product manager for EUROIMMUN, a PerkinElmer company. EUROIMMUN is a global leader in autoimmune testing and an emerging force in infectious disease, allergy and molecular genetic testing. Its expertise and capabilities extend across immunology, cell biology, histology, biochemistry and molecular biology.