The importance of implementing a quality management system in the laboratory
Laboratory quality can be defined as accuracy, reliability, and timeliness of reported test results. To be useful, laboratory results must be as accurate as possible, all aspects of the laboratory operations must be reliable, and reporting must be timely. Some significant consequences of poor quality in the laboratory can include:1 unnecessary treatment or treatment complications, failure to provide correct treatment, delayed diagnosis, and unnecessary follow-up diagnostic testing. These consequences result in increased cost in time and work, as well as poor patient outcomes. Two facets of quality management in the laboratory are quality control (QC) and quality assurance (QA).
Quality control vs quality assurance
QC is defined by the International Organization for Standardization (ISO) as the part of quality management focused on fulfilling quality requirements.2 QC requirements are mandated by regulatory agencies and must be followed by the laboratory to fulfill accreditation requirements set forth by those regulatory agencies. ISO’s standard Medical laboratories–requirements for quality and competence (ISO 15189: 2012) delineates a set of requirements the laboratory must fulfill to pass accreditation requirements set forth by accrediting agencies.3 Some accrediting organizations use the information in ISO 15189 to guide the inspection and accreditation process. While the ISO standard provides broad-based guidance on implementation of regulatory requirements, other standards are available for the laboratory that gives step-by-step detailed guidance on how to fulfill these requirements.4
QA is the part of quality management focused on providing confidence that quality requirements will be fulfilled.2 Implementation of a quality management system (QMS) is an effective way to ensure QC and QA goals are met and maintained in the laboratory.
The quality management system
Laboratory error can be minimized by the implementation of an effective quality management system (QMS). A laboratory QMS is a systematic, integrated set of activities to establish and control the work processes from preanalytical through postanalytical processes, manage resources, conduct evaluations, and make continual improvements to ensure consistent quality results.5 All aspects of the laboratory operation—including the organizational structure, processes, and procedures—need to be attended to in a QMS. In the U.S., a QMS is a Clinical Laboratory Improvement Amendment (CLIA) requirement.6 A laboratory that implements the QMS model can expect the following outcomes:4 better ability to reduce or eliminate error, higher likelihood of meeting customer expectations, more effective and efficient operations, and greater potential for successful governmental and accreditation assessments.
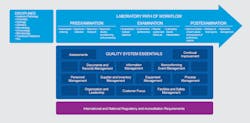
The quality management system model for laboratory services, shown in Figure 1, below, organizes all laboratory activities into 12 quality system essentials.
The quality system essentials
The quality system essentials (QSE) are the building blocks of a QMS. All 12 QSEs must be included in the QMS to assure accurate, reliable, and timely laboratory results. The 12 QSEs may be implemented in the order that is most effective for each individual laboratory. Implementing a QMS does not guarantee an error-free laboratory, but it can help maintain a high-quality laboratory that detects errors and prevents them from recurring.1
The 12 quality system essentials are:4
- Organization: Describes key leadership responsibilities that are integral to a laboratory’s success in achieving and maintaining a systematic approach to quality and meeting regulatory, accreditation, customer, and internal requirements.
- Customer focus: Describes the need to design work to meet the expectation of laboratory customers. It also describes methods for seeking customer input to confirm that expectations are continually met.
- Facilities and safety: Provides information about the maintenance and safety programs needed to support the laboratory. The laboratory needs to establish and maintain a facility that provides adequate space, workflow, and environmental conditions to support the quality of work and safety for all staff, in compliance with requirements.
- Personnel: Describes obtaining and retaining an adequate number of qualified, well-trained, and competent laboratory staff to perform and manage the activities of the laboratory.
- Purchasing and inventory: Describes agreements that the laboratory has with customers and outside vendors to ensure that specified requirements for
critical supplies and services are consistently met. - Equipment: Describes selection and installation of equipment, equipment maintenance and calibration, documentation of equipment-related problems, and record maintenance.
- Process management: Describes processes directly and indirectly related to the laboratories path of workflow to meet requirements and maintain efficient use of resources.
- Documents and records: Describes the creation, management, and retention of the policy, process, and procedure documents for the QSEs and path of workflow.
- Information management: Provides guidance for managing the information generated and entered into laboratory recordkeeping systems (e.g., patient demographics, examination results and reports, interpretations).
- Nonconforming event management: Describes processes for detecting and documenting nonconformances, classifying nonconformances for analysis, and correcting the problems they represent.
- Assessments: Describes the use of external and internal monitoring and assessments to verify that laboratory processes meet requirements and to determine how well those processes are functioning.
- Continual improvement: Describes mechanisms for identifying opportunities for improvement and developing a strategy to continue this improvement.
Conclusion
Implementing a QMS in the laboratory is essential to providing quality test results and patient care and is a requirement for passing and maintaining laboratory accreditation in the U.S. under CLIA regulations.
REFERENCES
- World Health Organization. Laboratory quality management system: handbook, Version 1. Geneva: World Health Organization, 2011.
- ISO. Quality management systems. Fundamentals and vocabulary. ISO 9000. Geneva, Switzerland: International Organization for
Standardization; 2015. - ISO. Medical laboratories. Requirements for quality and competence. ISO 15189. Geneva, Switzerland: International Organization for
Standardization; 2012. - CLSI. Quality Management System: A Model for Laboratory Services; Approved Guideline—Fourth Edition. CLSI document QMS01-A4. Wayne, PA: Clinical and Laboratory Standards Institute; 2011.
- Carey RB, et al. 2018. Implementing a quality management system in the medical microbiology laboratory. Clin Microbiol Rev 31:e00062-17. https://doi.org/10.1128/CMR .00062-17.
- U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services. 2003. Medicare, Medicaid and CLIA programs; laboratory requirements relating to quality systems and certain personnel qualifications. Final rule. 42 CFR 493. Fed Regist 68:3640 –3714.
About the Author

Joanne Christopher
MA, ELS
is a content specialist for the Clinical and Laboratory Standards Institute (CLSI). She is a board-certified life sciences editor and a member of the American Medical Writers’ Association (AMWA) and the Board of Editors in the Life Sciences (BELS). Joanne has been writing for the healthcare industry for 15 years.