Laboratory training and competence assessment in the era of COVID-19
COVID-19 brought many challenges to the laboratory in 2020 and will continue to do so in 2021. The SARS-CoV-2 tests currently being used under emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) will evolve to have improved performance, and the manufacturers will obtain official regulatory clearance or approval. Other tests may no longer be available once their EUA expires, and laboratories using those tests will have to implement different tests. With these and other changes coming, it is as important as ever to have a robust, efficient, and effective training and competence assessment program for current and new laboratory staff members.
This article provides information on best practices for training and competence assessment, detailing a structured approach that can help laboratories establish a planned and systematic culture of training and competence assessment that meets regulatory and accreditation requirements. Given that people are the most valuable resource in an organization, an effective training and competence assessment program will ensure that the personnel are knowledgeable and competent to do their assigned jobs. Effective training1 and competence assessment programs:
- Ensure personnel performance results in consistent, predictable, and high-quality outcomes.
- Ensure performance of assigned job tasks remains constant.
- Verify that staff members have and can demonstrate the necessary knowledge, skills, and behaviors to perform their respective duties.
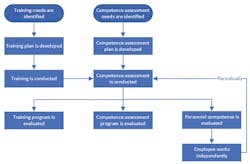
Training and competence assessment are two separate and distinct activities. Training focuses on teaching the skills needed for a job, along with the work processes and procedures for job tasks, including quality, safety, IT systems, and other responsibilities. Competence assessment, on the other hand, follows training, and assesses whether the training was effective, and the trainees are ready to function in their work environment. Ongoing competence assessments ensure that personnel continue to demonstrate the necessary knowledge, skills, and practices to remain effective in their jobs. Figure 1 shows the relationship between training and competence assessment.
Training programs
Training cannot typically be merely having a person read a procedure and sign off on a checklist. Procedures do not happen in isolation and are part of a process of many interlinked procedures for distinct activities that need to occur in a specific order. Training needs to be provided on work processes and include all the procedures that apply to any given process. Laboratories need to identify their various processes and associated procedures for all the work that is performed in the lab. They need to understand how many people are involved in each process and who performs each task. Once the processes, procedures, and people are understood, a training plan can be developed that takes into account the current knowledge and skill level of the personnel involved with any given process.
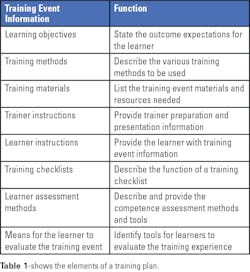
Determining the learning objectives is a very important first step. These objectives identify what needs to be learned and the activities that need to be demonstrated by the learner after the training session. The objectives should define specific, observable, or measurable behaviors.
Multiple training methods can be used, as deemed appropriate for each objective. Methods can include, for example, self-study materials, demonstrations, lectures, videos, computer-based exercises, supervised practice sessions, and testing of samples.
Training materials can include items such as operator’s manuals, procedures, handouts, practice materials, and others as needed for the specific training. Instructions for trainers and learners should be created to minimize any variability between instructors and to guide learners as they proceed through the training. The instructions should also include a list of materials needed for any demonstrations or practice sessions for learners. Using training checklists can help a learner complete each training activity successfully and serve as a record of completed training.
At the completion of the training, a learner’s knowledge can be assessed by quizzes (oral or written), observation of activities, or a record review. And finally, a learner should have an opportunity to evaluate and provide feedback on the training event. It is important to capture whether a learner’s expectations for the training have been met and whether the learning objectives have been achieved.
The training environment must be conducive to learning, and the learner needs to practice in a “safe” environment, where they are not punished for mistakes, they have time to learn and reflect, and they can make conclusions from the information and activities learned. Whenever possible, the training should take place in the work area. For nontechnical training, a conference room will suffice. No matter where the training is conducted, the learner needs access to private conversation space for asking questions and expressing concerns.1
Competence assessment programs
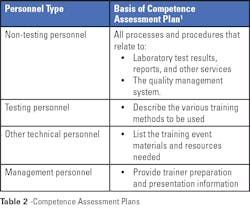
Competence assessment is required by regulatory and accrediting organizations; it is a good quality practice that should be an ongoing part of a laboratory’s culture. As with most programs, the first step is to develop a comprehensive plan. In the case of planning for competence assessment, the plan needs to be based on and include activities for each job title, including quality management activities as appropriate. Criteria that define acceptable performance or a passing score need to be determined before implementing the program. All materials used in the competence assessment are subject to document control.
Competence assessment best practices include, as appropriate for each work process or procedure:1
Directly observing routine work processes and procedures, including applicable pre-examination and post-examination processes and applicable safety practices. This is an objective means of assessing performance, especially for routine work, which should focus on high-risk process activities and procedure steps.
- Directly observing equipment maintenance and function checks.
- Monitoring the recording and reporting of examination results, including identifying any critical results, errors, or other possible issues. Such monitoring should include reviewing test results, QC records, calibration records, preventive maintenance records, any troubleshooting records, proficiency testing results, and any corrected or amended reports.
- Reviewing work records, such as work cards, log sheets, and computer data entry.
- Examining specially provided samples, such as previously examined samples, interlaboratory comparison (i.e., proficiency testing [PT]) materials or split samples, and in some cases, QC material.
- Assessing problem-solving skills, especially through simulations that can determine whether staff members problem-solve within their knowledge and skill level, escalating issues when appropriate.
Once the competence assessment plans are developed and approved, the assessments can be conducted. Assessments should be scheduled periodically based on requirements from regulatory or accrediting organizations. The best practice is to build ongoing competence assessment schedules into the laboratory routine, with consideration of the usual path of workflow. Not all personnel need to be assessed at the same time. Schedules should allow the daily workflow to continue without being disrupted by the assessments.
Records of all competence assessment activities need to be retained in individual personnel files. These records should include the names of the assessor and the person being assessed, as well as indicating what was reviewed or observed, when it occurred, and the outcome. The objective evidence of passing the competence assessment must be retained (direct observation checklists, testing data, etc.). It is important to ask the people who pass a competence assessment to sign a statement in which they commit to using the skills needed to perform their duties in the workplace. The assessment is not a group activity, as it is assessing individual skill, not group knowledge.
How to begin
A new training program is more likely to be accepted when a new change is occurring because staff has not already been trained by an older training method.
New training programs are best started when, for example, any of the following changes occur:
- A new or changed process is implemented.
- New instrumentation or tests are brought into the lab.
- Regulatory or accreditation requirements change.
- A process is problematic and needs to be improved.
- New quality improvement tools are implemented.
COVID-19 specific training
Training programs for COVID-19 testing need to include instruction about sample collection, storage, and transport; performing a test properly; and reporting the results. Each of these activities are likely to be performed by different people, and the development of a comprehensive training plan to ensure all personnel receive their appropriate training is needed. Training in this circumstance can be especially challenging due to the number of people needing training in a short amount of time.
Sample Collection:2 It is important to collect, transport and store a sample properly for accurate and reliable test results, and training for personnel performing sample collection is essential. When providing collection of nasal swab samples for viral testing, laboratories need to be sure they follow their test manufacturer’s instructions.
The manufacturer’s package insert for the SARS-CoV-2 test contains specific information about the swab types authorized for use with the test. For viral testing, rayon or synthetic polyester fiber-tipped swabs are recommended, and calcium alginate or cotton swabs are not recommended. Nasopharyngeal samples are typically collected using swabs with fine, flexible wire shafts. Wooden-shafted swabs are not recommended because they can contain toxic or inhibitory materials and can also absorb some of the specimen volume.2
Typical directions for use of a nasal swab are:2
- Pass a flexible, fine-shafted swab into the nostril.
- Rotate slowly for five seconds to absorb secretions.
- Immediately place swab into a vial of the appropriate viral transport media. (Drying will result in loss of viral viability.)
- Repeat for the other nostril using a fresh swab. Place both swabs into the same transport tube.
Sample Storage and Transport: All sample storage and transport instructions are given in the manufacturer’s package insert and need to be followed to ensure accurate test results. Training on these procedures should be given to all personnel who handle the samples.
Testing: Whether the tests are performed at the point-of-care or in the laboratory, laboratory management should ensure that all staff members who perform testing receive proper training. They need to be trained on:
- Setting up instruments and reagents before testing
- Calibrating instruments and running QC samples
- The testing procedure
- Obtaining and reporting the test results in the laboratory information system or patients’ charts
- Knowing if the test worked properly
- Knowing what could go wrong with the test and how to avoid errors
- Performing any necessary maintenance
- Proper reagent, calibrator, and QC handling and storage
Rapid tests3 are typically simple to use and provide results within 30 minutes or less. A number of point-of-care tests for SARS-CoV-2 have been authorized for use, with varying sensitivity, specificity, and turnaround time. Users should be aware of a test’s sensitivity and specificity and ensure that providers report results accordingly. Rapid test accuracy depends greatly on specimen quality and test performance. Low test sensitivities can make false negatives common, and negative rapid test results may require confirmation by a more sensitive test method.
Nucleic acid amplification tests are more sensitive but also easily subject to contamination. It is very important that personnel performing these tests are trained in all aspects of testing, sample handling, and how to avoid potential cross-contamination.
Reporting requirements for COVID-19 tests can include reporting not only into the laboratory information system or patient’s chart, but also to local or regional health authorities for infection rate tracking. Training of each of these requirements needs to be provided for the appropriate personnel.
Summary
In summary, training for COVID-19 testing can certainly seem daunting at first, especially since it affects every area of the laboratory and all levels of laboratory workers. If the laboratory has a well-functioning training and competence assessment program already established, it is a matter of developing the specific materials needed to train staff members in the processes and procedures for each job function. While this remains a big task to undertake, a systematic approach can ensure that people are properly trained in the activities that they need to do for their job. Many test manufacturers have developed training programs and materials specific for their tests, and laboratory managers should not hesitate to reach out to test manufacturers for assistance with developing training programs.
References
- Clinical and Laboratory Standards Institute. Training and Competence Assessment – Fourth edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2016. CLSI document QMS03.
- Clinical and Laboratory Standards Institute. Viral Culture – First edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. CLSI document M41.
- Clinical and Laboratory Standards Institute. Point-of-Care Testing for Infectious Diseases – First edition. Clinical and Laboratory Standards Institute; 2020. CLSI document POCT15.
About the Author

Luann Ochs, MS
is Product Development Manager at the Clinical and Laboratory Standards Institute.