Forecasting supplies for laboratories and hospitals, post pandemic, is a complex task, involving a complicated bag of tricks. Suppliers are also gravely affected by the shortages, always conscious of the needs of their customers.
The Center for Biologics Evaluation and Research (CBER), within the FDA that regulates biological products for human use under applicable federal laws, identifies shortages of biologics as a threat to public safety.
“CBER’s Office of Compliance and Biologics Quality (OCBQ) directs the CBER-regulated product shortage program, which includes product discontinuations. [Currently,] shortages of drugs and biologics pose a significant public health threat, delaying, and in some cases even denying, critically needed care for patients.”1
With significant challenges, due to shortages, supply forecasting and acquisition require laboratories and hospitals to examine a number of different categories in order to acquire necessary supplies to effectively complete the tasks at hand.
Causes of shortages
The FDA is aware of the shortages, brought on by the pandemic, and explains the causes and possible other scenarios.
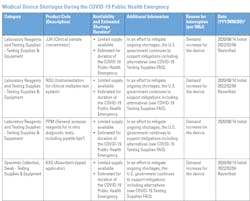
In a January 2022 news release entitled Blood Specimen Collection Tube Conservation Strategies - Letter to Health Care and Laboratory Personnel, the U.S. Food and Drug Administration (FDA) stated that it “is aware the United States is experiencing significant interruptions in the supply of several blood specimen collection (blood draw) tubes because of an increase in demand during the COVID-19 public health emergency and recent vendor supply challenges. The FDA is expanding the medical device shortage list to include all blood specimen collection tubes.”2
In another article, CBER-Regulated Products: Possible Causes of Shortages, the FDA introduces the possibility of additional causes for supply shortages.
“Quality problems at the manufacturing facility are the most common causes of CBER-regulated product shortages. Other causes of product shortages include increased demand, corporate delays in manufacturing or shipping, distribution disruptions, production changes, unavailability of component materials, new indications, decisions to discontinue the product, or natural disasters,”3 the FDA article states.Laboratories/Hospitals:
Inventory management
Focusing on inventory management, storage and distribution, introduction of new products, the ever-changing COVID-19 pandemic outlook, and best practices that include future pandemic readiness, laboratories forge ahead with the purchasing of supply inventory and assembling a stockpile of supplies, ever mindful of unexpected events and emergency situations, similar to the COVID-19 outbreak.
Implementing the KanBan principles, a visual system that manages inventory control, Bower and her laboratorians are able to diagnose the potential upcoming hardships related to the ordering of necessary supplies. A Kanban bin system for inventory management, uses two bins in your supply area to visually see when it’s time for restock. In general. When the first bin of materials or work-in-process is empty, it’s time to reorder or restock.
Storage and distribution
Determining and forecasting, the PAR levels of supplies within the laboratories require the utilization of data in order to determine test volumes.
Bower states, “The laboratory is responsible for determining our PAR levels for lab supplies. This is done using data from our Slicer Dicer app within Epic (to determine test volumes) and also by reviewing past ordering frequencies.”
Introduction of new products
When introducing new products into the laboratories and hospitals, procedures must be followed to ensure a smooth transition for all members of the staff.
According to Bower, Mary Greely Medical Center is lucky to have a purchasing agent who worked in the laboratory, prior to her current position, with a good understanding of lab supplies. When asked about the procedure for introducing new products, Bower said, “Under normal circumstances, when a brand-new product is introduced, laboratory staff try the product and then fill out an evaluation form, prior to integrating the product. Their input is instrumental to the process.”
Bower said an area where the laboratory was forced to integrate new testing products and supplies involved COVID-19 testing. She stated, “This affected nursing as well as the lab. Our microbiology section head worked tirelessly to ensure lab staff were trained very quickly…because we still had to meet CLIA regulations with respect to introducing new tests. To keep all lab staff apprised of ongoing changes, we formed a channel in MS Teams called “COVID” and also devoted one of our white boards in the lab to just COVID related updates. We also communicated change of testing supply information to the nursing leaders of all affected departments.”
Changes due to COVID-19 pandemic
The COVID-19 pandemic illuminated supply shortages, wreaking havoc on laboratories and hospitals. Fortunately, the evolution of hyper-awareness and ingenuity became the norm for those labs that continued to provide necessary testing during a most difficult time.
COVID-19 brought unprecedented challenges to clinical laboratories. While U.S. labs strove to provide quality and accurate test results in the face of 2020’s adversity, the uncertainty and lack of supplies were a significant hurdle, hindering day-to-day laboratory operations and the ability to increase testing capacity.
According to the article, Supply Shortages Impacting COVID-19 and Non-COVID Testing, “the uncertainty and lack of supplies were a significant hurdle, hindering day-to-day laboratory operations and the ability to increase testing capacity.”4
“In 2020, ASM partnered with the Association of Supply Chain Management to collect testing supply status for both COVID-19 tests, as well as other microbiological tests to highlight, and ultimately alleviate, these debilitating supply chain issues. Starting Sept. 11, 2020, ASM began independently collecting shortage data directly from clinical labs using the Clinical Microbiology Supply Shortage Collection (CMSSC) tool, drawing attention to the data provided by laboratory directors, and practicing clinical microbiologists without external influence. This data reflects shortages of medium, reagents, collection devices and consumables that significantly impacted day-to-day testing for both COVID-19 and other infectious diseases during the time period of Sept. 11, 2020 to Jan. 15, 2021.”4
“147 CLIA-certified labs responded to the survey across the U.S. The CMSSC tool results also show the following non-COVID-19 shortages:
- 35.1% of labs have a shortage of supplies for the molecular detection of sexually transmitted infections.
- 47.5% of labs have a shortage of supplies for detection of routine bacteria (including the bacteria causing strep throat, pneumonia, bronchitis, and urinary tract infections).
- 29.4% of labs have a shortage of supplies for mycobacteria testing (including supplies for tuberculosis (TB), Buruli ulcer and pulmonary nontuberculous mycobacterial disease testing).
- 8.8% of labs have a shortage of supplies for routine parasite testing.
- 19.4% of labs have a shortage of supplies for routine fungal testing (ranging from superficial, localized skin conditions to deeper tissue infections to serious lung, blood (septicemia) or systemic diseases).”4
When asked if her laboratory’s process of acquiring and/or integrating supplies has changed since the COVID-19 pandemic, Mary Greeley’s Bower replied, “We still tend to utilize our KanBan system for most supplies. However, any item which has been subject to a backorder receives special treatment. We may order more than our usual number of the product, or order more frequently, depending on the advice of our buyer in the purchasing department. We have become much more “tuned in” to laboratory news with respect to national shortages.”
Best practices and future pandemic readiness
Having experienced the COVID-19 pandemic, and the effects of supply shortages, the idea of a preparedness plan seems likely, and necessary, at the same time. Mary Greeley Medical Center is among those laboratories that is continuously working to institute such a plan, based on the awareness brought about through the COVID-19 pandemic. Additionally, the pandemic shown a light on collaboration with borrowing and sharing supplies among labs and hospitals and creating partnerships.
“We have not yet determined a definite plan for future pandemic readiness as it relates to supplies. However, one thing that became very apparent to us was that our collaborative relationships with nearby clinics and hospitals was instrumental to effectively managing unexpected shortages. We were able to loan blue top tubes to a very large hospital who otherwise would not have been able to get them at all. When we ran perilously low on purple and green top tubes, we received small loans of tubes from several other institutions that helped us make it to the next shipment,” Bower said.
To help laboratories deal with supply shortages, The Choosing Wisely program was created, which is an initiative of the ABIM Foundation that focuses on avoiding unnecessary medical tests, treatments, and procedures.5
“Programs such as Choosing Wisely, and other laboratory medicine stewardship guidelines, have been designed around the patient-centric and fiscally prudent principle of reducing testing that adds no value to patient care, and that even may be associated with increased risks…COVID-19 supply chain issues have clearly created a healthcare crisis, including for the practice of laboratory medicine. But this crisis presents laboratorians with a golden window of opportunity to initiate or strengthen our effective test utilization and laboratory stewardship efforts, using Choosing Wisely and other guidelines as a foundation for engaging in interdisciplinary organizational discussions.”6
Suppliers/Vendors:
Managing the needs of laboratories
Lee H. Hilborne, MD, MPH, FASCP, DLM(ASCP)CM, and colleagues, in an article entitled, Laboratory Supply Shortages: Turning Crisis to Opportunity, wrote, “as the pandemic continues to ravage the global medical community, supply chain issues have introduced new challenges that extend far beyond COVID-19 testing. Shortages of specimen tubes, personal protective equipment, and other common laboratory consumables threaten access to all aspects of diagnostic testing.”
Vendors, as suppliers for laboratories and hospitals, also have been affected by the upheaval occurring, due to shortages and the pandemic. Fortunately, not all suppliers have experienced, or have been negatively affected by current pandemic-type shortages.
As a way to explain, Noel referred to the company’s “innovative test strip manufacturing process…and automated manufacturing lines that can produce tens of millions of tests per month.”
While Noel’s experiences regarding the on-going supply chain in the pandemic and post-pandemic timeframe are notable, they are not the norm, for suppliers. Because of the long-standing difficulties brought on by the shortages, government agencies have stepped in with suggestions for buffering such adversities.
Recommendations
For the time being, while pandemic shortages still exist, the FDA has provided recommended strategies for the conservation of supplies.
The FDA recommends healthcare providers, laboratory directors, phlebotomists, and other personnel consider the following conservation strategies to minimize blood collection tube use and maintain quality and safety of patient care2:
- Only perform blood draws considered medically necessary.
- Remove duplicate test orders to avoid unnecessary blood draws.
- Avoid testing too frequently or extend time intervals between tests whenever possible.
- Reduce tests at routine wellness visits and allergy testing only to those that target specific disease states or where it will change patient treatment.
- Consider add-on testing or sharing samples between laboratory departments if previously collected specimens are available.
- If you need a discard tube, use a tube type that has a greater quantity available at your facility.
- Consider point of care testing that does not require using blood specimen collection tubes (lateral flow tests)
References:
- CBER-Regulated Products: Shortages and Discontinuations. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/cber-regulated-products-shortages-and-discontinuations
- UPDATE: Blood Specimen Collection Tube Conservation Strategies - Letter to Health Care and Laboratory Personnel. https://www.fda.gov/medical-devices/letters-health-care-providers/update-blood-specimen-collection-tube-conservation-strategies-letter-health-care-and-laboratory
- CBER-Regulated Products: Possible Causes of Shortages. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/cber-regulated-products-possible-causes-shortages
- Supply Shortages Impacting COVID-19 and Non-COVID Testing. https://asm.org/Articles/2020/September/Clinical-Microbiology-Supply-Shortage-Collecti-1
- ABIM Foundation, https://www.choosingwisely.org/
- Lee H Hilborne, MD, MPH, FASCP, DLM(ASCP)CM, Greg Sossaman, MD, MACSP, Barbara Caldwell, MS, MASCP, MLS (ASCP)CM, SHCM, et al. Laboratory Supply Shortages: Turning Crisis to Opportunity. American Journal of Clinical Pathology, aqac035, https://doi.org/10.1093/ajcp/aqac035.
Gail Castanho, MLO Managing Editor