The cornerstone of effective stewardship is in the microbiology lab
Earning CEUs:
For a printable version of the August CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
AUGUST LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss the positive and negative attributes of the use of antibiotics.
2. Define antimicrobial resistance (AMR) and the factors that have caused it.
3. Recall statistics of AMR including usage, deaths, and
associated healthcare costs.
4. Discuss how effective Antibiotic Stewardship Programs (ASPs) can be accomplished and the main goals of an ASP.
Over the past century, the number of deaths due to infectious diseases has decreased dramatically through the extensive use of antibiotics. Antibiotics have also made a number of “modern-day medical miracles” possible, such as organ transplantation, cancer chemotherapy, treatment of preterm babies, and major surgeries.
Without antimicrobials, the infections associated with these diseases and medical interventions would be extremely frequent and potentially fatal. Because antimicrobials are so effective, they have been massively overused to treat both humans and animals. The development of antimicrobial resistance (AMR) is accelerated by the selective pressure exerted by the widespread use of antimicrobials.
Cases of AMR were usually detected in hospitals, but they have now spread outside these settings to the community. Some bacteria have become resistant to multiple drugs, leading to situations where there are no treatment options left to fight the patient’s infection. Until just recently, a lack of new antibiotics in the development pipeline further compounded the situation.
Antimicrobial resistance phenomenon
AMR is a phenomenon which describes the non-susceptibility of microbes (bacteria, fungi, viruses, and parasites) to antimicrobial drugs. As microbes evolve over time, whether exposed to antimicrobials or not, they can develop resistance mechanisms that allow them to survive exposure to antimicrobials. AMR is a natural anomaly which confers a survival benefit to microbes, but is increased and accelerated by their exposure to antimicrobials through their misuse or overuse in human medicine, by inadequate infection prevention and control, from poor hygiene and sanitation practices, and due to unnecessary antibiotics used in agriculture and farm animal production. In addition, as a result of globalization of populations, animals and food, these antibiotic-resistant so-called “superbugs” can spread rapidly and easily between cities, countries, and continents.
The infections caused by resistant pathogens are becoming increasingly harder to treat, with many reports of patients with no antibiotic options left to treat their infection. A recent global study from a team at Johns Hopkins University showed that the problem has been fueled by an astonishing 65 percent increase in antibiotic use between 2000 and 2015.1 The status quo is not an option, as the need has never been more urgent because of the increase in the incidence of AMR worldwide. The number of antimicrobial R&D programs has declined steadily over the past 30 years.2 Fortunately in recent years, several new agents have achieved U.S. Food and Drug Administration (FDA) approval, and several more are under late-phase development. However, without prudent use of these new compounds, their efficacy may be short lived.
The cost of non-action is enormous and will grow, putting patients at risk “with a return to a situation where 40 percent of the population die prematurely from infections we cannot treat” and making medical practices in some patients (chemotherapy, organ transplantation, some otherwise routine surgeries) highly risky.
Annul deaths attributable to AMR now exceed 700,000 globally and are predicted to reach 10 million per year by 2050.3 This is a conservative estimate, since that report only considered a relatively small sub-group of resistant human pathogens. Moreover, the World Bank warns about the economic consequences of AMR, in particular a decrease in annual global GDP between 1.1 percent and 3.8 percent by 2050. In low-income countries, AMR could increase extreme poverty, with an additional 28.3 million people affected by this issue by 2050.
A global emergency
Nearly all of the world’s largest governmental and public health organizations have identified AMR as a global emergency, including the World Health Organization (WHO), U.S. Centers for Disease Control and Prevention (CDC), G20, United Nations General Assembly, and the European Commission. In the United States, the CDC and Centers for Medicare and Medicaid Services (CMS) highly recommend all U.S. hospitals implement antimicrobial stewardship programs (ASPs). Effective January 2017, the Joint Commission hospital accreditation body (JCAHO) required all hospitals and nursing care centers have Antibiotic Stewardship Programs (ASPs).
ASP efforts outside of the U.S. include:
- The United Kingdom’s five-year national strategy (2013-2018) for tackling AMR includes optimizing antibiotic prescribing through stewardship.4
- The German Society for Infectious Diseases published an evidence-based guideline in December 2013 requiring successful implementation of ASPs in German hospitals.5
- In South Africa, antimicrobial surveillance and reporting, antimicrobial stewardship, and improved infection prevention and control form the three pillars of the national AMR strategy framework for the years 2014-2019.
Combatting AMR
Diagnostics play a major role in combating AMR on three different levels:
- To achieve optimal diagnosis and therapy of the individual patient.
- To achieve optimal cost-efficient functioning of healthcare systems.
- To promote the public health and improve life for patients with infectious diseases worldwide.
With so much focus on the threat of AMR, we too often only focus on the need for novel antibiotics, but forget that we must also take urgent steps to sustain the efficacy of the antibiotics we already have in order to preserve these life-saving drugs for future generations. To do this we need innovative and rapid diagnostics, and we need to use them properly.3
In fact, diagnostics have done much to curb the spread of resistant organisms through more rapid technological advancement than new antibiotics, which take many years to research, develop, and gain regulatory approval. The common cornerstone of every effective ASP is the microbiology lab. While many U.S. hospitals have implemented mandatory ASPs to reduce the misuse and overuse of these drugs, many hospitals have yet to launch robust and effective stewardship teams and programs because the lack the expertise, funding, time—or because it simply is not a priority among hospital management.
As healthcare providers, we must continue to remind hospital administrators that diagnostics provide nearly all of the basic information used in about 70 percent of clinical decisions. Unfortunately, the microbiology laboratory does not stand among hospital stakeholders as having the biggest influence in antibiotic usage, but this must change for us to defeat AMR. It is vital that hospital administrators realign their perception of the microbiology lab as simply a cost-center and begin to see if for what it really is: The cornerstone of effective stewardship. We must rapidly advance our ASPs nationwide and to do this hospital administrators are encouraged to do the following:
- Consider in vitro diagnostics as a fundamental tool in the fight against AMR and of all ASP efforts;
- support the transformation of the microbiology lab role in ASP into a vital component; and
- develop educational programs around the importance of diagnostics in combatting AMR and their pivotal role within ASP efforts, for both clinicians and their patient populations.
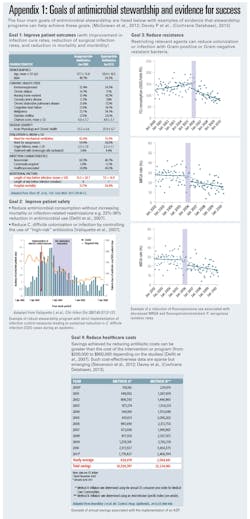
While implementing effective stewardship takes effort and resources, stewardship provides rapid cost savings, in addition to improving patient care. For example, ASPs can dramatically reduce pharmacy expenditures on overuse and misused antibiotics. (Appendix 1)
With the microbiology laboratory and the pharmacy working in close partnership, rapid therapeutic decisions can be made and personalized based on the latest diagnostic information. By identifying the most appropriate antimicrobial therapies, stewardship programs are essential to improving patient outcomes and patient safety, preserving the efficacy of existing antimicrobials, and reducing resistance and healthcare costs. One of the cornerstones of high-performing stewardship programs is the proper use of rapid diagnostic platforms.
The founding of PACCARB
Noting that the rise of antibiotic-resistant bacteria represents a serious threat to public health and the economy, President Barack Obama created the President’s Advisory Council on Combating Antibiotic Resistant Bacteria (PACCARB) in 2014 with the Executive Order for Combating Antibiotic-Resistant Bacteria.
Detecting, preventing, and controlling antibiotic resistance requires a strategic, coordinated, and sustained effort. The federal government works domestically and internationally to detect, prevent, and control illness and death related to antibiotic-resistant infections by implementing measures that reduce the emergence and spread of antibiotic-resistant bacteria and help ensure the continued availability of effective therapeutics for the treatment of bacterial infections.
The Advisory Council provides advice, information, and recommendations to the Secretary regarding programs and policies intended to support and evaluate the implementation of U.S. government activities related to combating antibiotic-resistant bacteria.
The PACCARB consists of 30 members, including 15 voting members that are special government employees, five non-voting liaison members representing their respective organizations, and 10 regular government employees representing the Department of Health and Human Services (HHS), the Department of Defense (DoD), and the United States Department of Agriculture (USDA). The members are supported by a Designated Federal Official and other staff members within HHS.
REFERENCES
- Superbug breakthrough. HeraldLIVE. https://www.heraldlive.co.za/news/world/2018-04-26-superbug-breakthrough/. Published 2018. Accessed June 19, 2019.
- Drive-ab.eu. http://drive-ab.eu/wp-content/uploads/2014/09/Rex-JH-2015-04-30-IFPMA-New-pathways-for-antibiotics-v1.3.pdf. Published 2019. Accessed June 19, 2019.
- Sfam.org.uk. https://sfam.org.uk/uploads/assets/uploaded/8382a639-75b9-4eb3-8ec71d8ac207325f.pdf. Published 2019. Accessed June 19, 2019.
- Johnson A, Ashiru-Oredope D, Beech E. Antibiotic Stewardship Initiatives as Part of the UK 5-Year Antimicrobial Resistance Strategy. Published 2015. Accessed June 19, 2019.
- de With K, Allerberger F, Amann S, et al. Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases. Published 2016. Accessed June 19, 2019.
About the Author

Christine Ginocchio, PhD, MT (ASCP)
serves as Vice President, Global Medical Affairs, bioMerieux, NC and BioFire Diagnostics, UT. She is an internationally recognized expert in the science, medicine, and policies of diagnostic technology.