Advanced clinical parameters: achieving efficiency with automated immature granulocyte count
The Complete Blood Count (CBC) is one of the most frequently ordered tests in the clinical laboratory. Its use ranges from screening for infection and anemia to monitoring disease progress and treatment. The CBC test often includes a leukocyte differential to determine the types and amounts of white blood cells (WBC) present. Recent cuts to reimbursement from the Centers for Medicare & Medicaid Services (CMS) have made it necessary for labs to increase efficiency—while retaining accuracy and rapid turnaround times (TATs)—when
performing this high-volume testing.
Immature granulocyte percentage
A great deal of research has focused on the utility of laboratory testing in the diagnosis of sepsis (a known or suspected infection plus systemic manifestations of infection) in the critically ill patient. Since the early 1990s, the guidelines for diagnosing sepsis, severe sepsis, and septic shock have undergone many significant alterations. However, among these guideline changes, the idea of infection being part of the underlying pathology of the condition has prevailed.1 From the idea that sepsis always begins with an infection, additional guidelines for diagnosing sepsis have focused on the immune system’s response to an infection, and in particular, a rise in the immature granulocyte (IG) percentage in patients with a normal total WBC count.
With these definitions in mind, a number of clinical studies have been conducted with the aim of utilizing laboratory testing, specifically the IG percentage, to rapidly and reliably detect infection. Initial and subsequent studies with severely ill inpatients reveal that:
- The IG fraction is a better predictor of infection than total WBC count2
- The IG fraction is comparable to the absolute neutrophil count (ANC) for predicting infection3
- An IG fraction of greater than three percent is a very specific (greater than 90 percent) predictor of sepsis.2
Increased IG fractions
More recent studies have focused on IG fractions in the outpatient population. In an extensive study performed in 2011, researchers at Boston Medical Center examined the clinical applications of the IG fraction in an outpatient population by relating increased IG fractions with particular illnesses and conditions relevant to outpatients.4
Numerous hematology analyzers have the capability to report a six-part automated differential that includes an IG fraction. The automated six-part differential data from these analyzers is usually reported in relative (percentage) and absolute (number of) units and includes the following cell types:
- Neutrophils (includes neutrophilic band cells)
- Lymphocytes
- Monocytes
- Eosinophils
- Basophils
- Immature granulocytes (includes promyelocytes, myelocytes, metamyelocytes)
Current six-part differential technology also uses flagging algorithms as part of an interpretive program to alert users to the possible presence of abnormal cells, as well as increases or decreases in the relative percentage of normal cells in the differential. Some of these flags are generated by the analyzer software, while others are triggered at user-defined cutoffs that are reflective of the laboratory’s Standard Operating Procedures (SOPs) and review criteria.
When immature granulocytes are present in numbers that exceed a user-defined limit, the analyzer will generate a flag message that reads “IG Present.” The appearance of this flag would prompt a smear review of the results that may include correlation with the patient’s history and diagnosis, or a manual examination of the blood film to verify the presence of immature granulocytes and any additional clinically significant features.
Smear reviews
In medical literature, much discussion debates the reliability of the 100-cell manual differential due to the minimal sample portion and the relatively low number of white cells counted.5 In contrast, a six-part “auto-diff” that includes the IG parameter, offers the benefit of higher precision (thousands of WBCs counted), a shorter TAT2 and the ability to detect possible infection.4 As a result, many labs are opting to perform smear reviews, as opposed to manual differentials, to search for features that enhance the reportable automated differential such as toxic white cell changes or other abnormal white cell morphology.
The hematology analyzer in the Core Laboratory at Rush University Medical Center (RUMC) in Chicago, IL processes 800-1,000 EDTA specimens per day. Result review is performed on all samples with interpretive messages generated by the analyzer. At RUMC, the “IG Present” flag will signal the slide preparation unit to generate a blood smear. The smear (slide) is then stained and reviewed to evaluate the accuracy of the automated differential.
Along with the “IG Present” flag, other flags may be generated by the analyzer such as “Abnormal Lymphocytes?” or “Blasts?” (Figure 1) These additional flags, on their own, or in combination, should also trigger a slide review by the laboratory technologist.
Since many of the samples processed in this lab are STATS from the cancer center, emergency department, or inpatient critical care units, reviews are often difficult and time consuming. While a number of these samples generate multiple flags, a significant amount flag only for the presence of immature granulocytes. The “IG Present” flag contributed to an overall review rate of 15 percent per day with the first shift performing the majority of the 80-100 smear reviews each day.
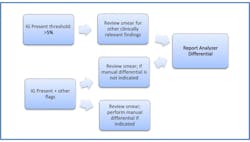
As previously stated, the “IG Present” message is user-defined and intended to be in line with the review criteria set forth in the individual laboratory’s SOP. At RUMC, the upper threshold for IG is set at 1.5 percent, meaning that any specimen with a relative IG count of 1.5 percent or greater will be flagged and sent to the differential bench for manual examination.
Following evaluation of the smear, the medical lab scientist will determine whether to report out the automated differential results as accurate or to perform a manual differential. This entire process often results in a significant workflow reduction as many Core Lab specimens have only the “IG Present” flag.
RUMC Core Lab review
In December 2015, the RUMC Core Lab performed a review of 637 auto-differential reports from the hematology analyzer that contained the “IG Present” flag. This was done at the request of the hematology supervisor, and in cooperation with the pathology staff, to assess whether the IG threshold to initiate smear review was set at an appropriate level. Two hundred white blood cell, red blood cell, and platelet IP messages, as well as flag combinations, were evaluated.
The study concluded that when only the “IG Present” message was generated—or “IG Present” combined with the “Left Shift?” message—the smear review did not yield any additional clinically significant information. As a further benefit, the IG result showed no evidence of interference from toxic granulation, which had been an issue with previous analyzer technology. It was determined that the IG review threshold could be increased from the current cutoff of 1.5 percent. Subsequently, the RUMC Core Lab raised the IG review threshold on the hematology analyzers to 5.0 percent. (Figure 2)
To determine the impact of raising the IG threshold on workload efficiency, RUMC Core Lab examined analyzer auto-differential reports from a seven-day period. Of the 601 manual review reports investigated during this time period, 264 reports generated an “IG Present” flag, with no other accompanying IP messages. Among the 264 auto-differential reports with only the “IG Present” message, 173 had an IG fraction of greater than 1.5 percent, but less than five percent, while 91 patient reports had an IG fraction of greater than five percent.
Of these 601 auto-differential reports, 434 reports had an IG fraction of greater than 1.5 percent and 167 reports had an IG fraction of greater than five percent. This meant that by increasing the IG threshold to five percent, the lab was able to reduce the number of auto-differentials submitted for manual review by approximately 30 percent per day. Raising the IG threshold had a significant, positive impact on workflow efficiency at the differential bench.
A timed study performed in the lab revealed that it takes about five minutes for the RUMC techs to perform each smear review. Raising the flagging threshold for IG eliminated approximately 25 smear reviews each day, saving almost 15 hours of tech time each week. The decrease in workload allowed the Core Lab techs more time to focus on problem patients, training activities and special projects.
Implementing the changes to the slide review policy was simple. Upon reviewing the study data, the hematopathologist was quick to approve the new flagging limit, noting that it may even be reasonable to raise the IG threshold to 10 percent in the future. The laboratory staff had been reporting automated IGs for more than 10 years, and IG was a trusted parameter among the techs and caregivers alike. Additionally, for the past number of years, the hematology techs were directed to validate the auto-differential (in lieu of performing a manual differential) whenever possible. This practice contributed to widespread acceptance of the IG result among caregivers and made it unnecessary to communicate the SOP changes to the medical staff.
In a lab that is operating 24 hours a day, seven days a week, and serving a 700-bed hospital with an ever-expanding outpatient population, the change in smear review procedures decreased TATs enabling technologists to spend more of their time assisting with other important bench work in the Core Lab. The IG parameter is a critical part in allowing RUMC Core Lab to manage a swelling workload and at the same time, deliver quality laboratory results to healthcare providers.
REFERENCES
- Singer M, Deutschman C, Seymour C, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016; 315(8):801-810.
- Buoro S, Mecca T, Vavassori M, et al. Immature granulocyte count on the new Sysmex XN-9000: performance and diagnosis of sepsis in the intensive care unit. Signa Vitae. 2015; 10(2):54-64.
- Nierhaus A, Klatte S, Linssen J, et al. Revisiting the white blood cell count: immature granulocytes count as a diagnostic marker to discriminate between SIRS and sepsis – a prospective, observational study. BMC Immunology. 2013; 14:8.
- Ansari-Lari MA, Kickler TS, Borowitz MJ. Immature Granulocyte Measurement Using the Sysmex XE-2100. Am J Clin Pathol. 2003; 120(5):795-799.
- MacQueen BC, Christensen RD, Yoder BA, et al. Comparing automated vs manual leukocyte differential counts for quantifying the ‘left shift’ in the blood of neonates. Journal of Perinatology. 2016; 36(10):843-848.
About the Author