Flow cytometry advances in the clinical and military arenas
Flow cytometry is so widely used nowadays for particle, tissue, and cellular research/analysis that we can almost take it for granted. We may also take for granted how much flow cytometry has improved over the years. Clinical laboratorians can analyze almost anything about generations of cells provided that they have the proper kits and reagents to do so. And the military has taken notice of this “analyze almost anything” aspect of flow cytometry, and has applied its use in military bio-detection and medicine models.
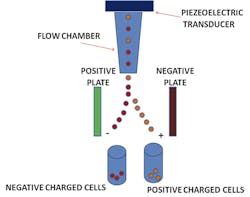
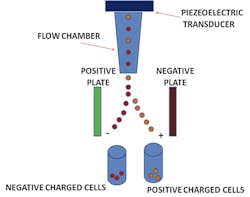
Flow cytometry was originally known as pulse cytophotometry.1 The name was introduced in 1976, about eight years after the patent was issued on this new kind of technology. In flow cytometry, particles are measured utilizing liquid and lasers on all types of cellular samples. The measured data found from particles passes through a liquid solution, and then impedance, along with fluorescence, stains the different components of the cells. The particles are suspended in a liquid and pass through a funnel, where a laser tags them for identification purposes. The light that passes through the particle is detected and determines the identifying factors for each particle measured.2 These identified components help to identify characteristics which are then used to diagnose specific diseases and disorders. (The beginnings of this methodology actually go back further; they originated with Wallace H. Coulter’s patented “Means for counting particles suspended in a fluid,” back in 1953.)
Clinical and research applications
Thanks to flow cytometry, we have been able to diagnosis more than 300 genetic disorders. Genetic disorders affect different components of the immune system and are pathologically, clinically, and immunologically heterogeneous. The advancement of genomics has enabled the discovery of 10 to 15 new genetic defects every year. The procedure is essential to analyze the immune system, quantitatively and functionally, to validate new genetic findings and provide accurate laboratory data to immunologists. Patients’ clinical symptoms do not always follow the textbooks to direct doctors to a single genetic defect, and flow cytometry is key in providing this vital information. It is used to assess protein expression, cell viability, apoptosis, cellular interactions, and cell enrichment. All that analytical data aids immunologists in diagnosing genetic disorders and making feasible prognoses.
Blood cells, both red cells and leukocytes, are traced back to a lineage, or early developmental stages. For example, monocytes are “bone marrow-derived leukocytes.”3
Additionally, leukocytes include neutrophils, lymphocytes, eosinophils, and basophils, among other components. These leukocytes sometimes are detected in earlier stages of development, depending on the medical criteria and patient status. Flow cytometry is an excellent tool in identifying those earlier cells within the patient blood sample. Microscopic examination enables the laboratorian to view and catalog the earlier cells. However, the use of flow cytometry both as a standalone and as a component of a more comprehensive laboratory analyzer allows laboratory staff to identify underdeveloped cells within a patient’s bloodstream. This enables clinicians to rule out or in multiple medical conditions. In addition, if a patient has a bone marrow disease that generates more promyelocytes or other earlier-stage cells, flow cytometry has proven to be beneficial in measurement of these cells due to their size and constituents.
Flow cytometry is also used frequently in medical research, as it enables tracking of cell generations depending on the study and research focus. It is increasingly being used to further specific research goals and projects, among them stem cell research. Stem cell monitoring is frequently used in modeling of therapeutic drugs, and flow cytometry is also used for tracking stem cells.
Military applications
Within the last decade, flow cytometry has been utilized in bio-detection for the military, in response to a threat whose true magnitude may have come to be broadly understood only during the Gulf War era and since. Flow cytometry is utilized in the U.S. Department of Defense’s BIDS (Biological Integrated Detection System), its standardized bio-agent threat matrix. This system uses a one to 10 um collection sample size, which is the typical size of most bio-warfare agents. With the addition of flow cytometry to BIDS, soldiers, sailors and airmen are able to detect and possibly identify a bio-warfare attack before it develops into a potentially serious situation.
Military personnel have been sent to almost every corner of the Earth, and a variety of medical conditions can occur as a result of those deployments. The use of flow cytometry in these cases may alleviate issues specific to military members in modern combat due to its regenerative and supportive roles. Advances in flow cytometry have included portable and hand-held flow cytometers4 utilized by both civilian and military laboratories. These flow cytometers are starting to make their way into clinical settings, enabling the laboratory to increase its footprint within the clinics in addition to its typical laboratory space.
That is a good example of the cross-pollination that is going on between clinical and military applications of flow cytometry. What is being learned in the military context is making its way back to the clinical laboratory. By enabling tracking of cell development within patient bloodstreams as well as its position in military research, flow cytometry has become a critical addition to the laboratory arsenal. As that arsenal increases, flow cytometry is an increasingly important piece of the diagnostic puzzle.
REFERENCES
- Barlogie B, Drewinko B, Johnston DA, Buchner T, Hauss WH, Freireich EJ. Pulse cytophotometric analysis of synchronized cells in vitro. Cancer Research. 1976;36:1176-1181.
- Kachel V, Messerschmidt R, Hummel P. Eight-parameter PC-AT based flow cytometric data system. Cytometry. 1990;11:805-812.
- Ziegler-Heitbrock L. Blood monocytes and their subsets: established features and open questions. Frontiers in Immunology. 2015;Aug 17. doi: 10.3389/fimmu.2015.00423.
- Portable flow cytometer by Handyem. BioOptics World. 7.16.12. http://www.bioopticsworld.com/articles/2012/07/portable-flow-cytometer-by-handyem.html.
MSgt Samantha Kunzelman, MT(ASCP), serves at the 88th Diagnostics & Therapeutics Squadron, 88th Medical Group, Wright Patterson AFB, OH.
SSgt Jillian A.N. Salazar serves at the 88th Diagnostics & Therapeutics Squadron, 88th Medical Group, 88th Air Base Wing, Wright Patterson AFB, OH.