What do we really need to know about platelets and the laboratory?
What is a platelet? The anatomic definition of a platelet is well established: According to MedicineNet.com, it is “an irregular, disc-shaped element in the blood that assists in blood clotting. During normal blood clotting, the platelets clump together (aggregate). Although platelets are often classed as blood cells, they are actually fragments of large bone marrow cells called megakaryocytes.”1 This definition, however, does not do justice to our rapidly expanding understanding of the platelet’s roles, functions, and laboratory applications.
What the numbers say
Laboratories with the ability to detect platelet function defects still tend to focus on identifying the two percent of the population that have heritable platelet function defects and von Willebrand Disease. The Scientific and Standardization Committee (SCC) of the International Society on Thrombosis and Haemostasis (ISTH) has published comprehensive guidance on the diagnosis of these inherited platelet function disorders. However, most laboratories don’t have all the listed resources to follow this guideline through the second of three tiers of testing.2
The Centers for Disease Control and Prevention’s (CDC) Registry for Blood Disorders data quantifies, with caveats, the reported annual rate of diagnosing patients with heritable platelet function disorders and von Willebrand Disease (vWD, types 1, 2 and 3 for purposes of this article).3 Those patients are likely to be referred to one of the 135 specialty centers (U.S. Hemophilia Treatment Centers Network) for care once identified. Based on self-reported data from these centers for 2012 through 2015, an average of 1,699, or about 13 patients per year per treatment center, are identified with heritable platelet function defects, and 49 percent of those patients are or become multi-year patients.
The three most common types of vWD are identified at a reported annual rate of about 5,118 cases or about 38 patients/year/center. About 52 percent of the vWD patients are or become multi-year patients.
What the lab can do
Therefore, we should largely forget what we think we know about the impact of most heritable platelet function defects on laboratory resources which are needed solely for detecting and diagnosing these dysfunctions. Instead, we should look at what the laboratory can do today, with established technologies, that will support multiple clinical specialties, improve patient care and outcomes, and provide the basis for the next generation of medical care—precision medicine. In other words, how can labs add critical value to the care process?
Platelets have critical roles in a number of basic physiologic processes. Hemostasis is one such process. Thrombosis is another. Others include inflammation, innate (natural) immunity, adaptive (acquired) immunity, tumor growth and metastasis, and the development of the lymphatic vessels.4 (It is likely that platelets have other roles which are not yet known.)
Platelet signaling is a multi-level, complex process involved in all their roles and functions. This signaling can result in the inhibition of highly specific or broad platelet functions, or activation of those functions.5 Because platelets are involved in these systemic physiologic processes, they become markers for, mediators of, and therapeutic targets in serious medical conditions and disease states.
The IVD industry may have oversold the benefits of individual platelet function tests to clinicians. Yale’s Dr. Harlan Krumholz challenged multi-journal advertising for a platelet inhibition test by stating that “no study has shown that a strategy guided by platelet aggregation testing produces better outcomes for patients.”6 Added to that is Dr. Schaef-Johns’ observation that “there is no perfect platelet function test…. Except for Light Transmission Aggregometry (LTA), no tests are endorsed by platelet function testing experts.”7
So what do we do? Do we turn the lights out and shut down the lab, as was suggested in a ‘Waugh’ Street Journal parody?8 No, but it is time to do things differently—things that matter to the clinician and the patient. Things that our laboratories can do now and do well. Things that are flexible, so that each laboratory can structure a service that suits the needs of its patient population and is within the laboratory’s functional capacity.
LTA is the gold standard for platelet function testing, including tests for the effects of drugs on platelet function. Methods for rapid, repeatable sample preparation (10-15 minutes) are available. Aggregometers are computer-driven, affordable, walk-away devices that can be comfortably operated by a hematology or other technologist. Results can be compared to local reference ranges and can be released through the pathologist or clinical pharmacist or directly to the clinician in less than 30 minutes. We must remember, though, that similar patients with the same diagnosis and treatment may very well give different results. That is the crux of why personalized medicine will be the next generation of all the inert-related medical disciplines, sciences, and technologies.
Patient non-adherence to medication plans is a major issue.9 As former U.S. Surgeon General C. Everett Koop was fond of saying, “Medicines do not work well in patients who do not take them.” Experiences with monitoring blood glucose and international normalized ratio (INR) in clinic and home settings have taught us that when data is expected to be generated and available, patient adherence improves, as does patient care outcome.10 Elwyn11 and colleagues provide the following reasons for patient noncompliance: 1) a lack of information about the advantages and disadvantages of the treatment; 2) the benefits of treatment are not obviously apparent; and 3) the psychological adaptation required to see oneself as in need of treatment is difficult to make. I would add a fourth: financial constraints. The ranges for non-compliance run from 20 percent to 80 percent. Mortality increases with decreasing adherence.
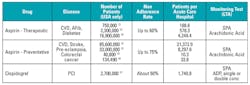
Table 1 suggests some ways that platelet-related testing can be used to personalize medicine. It brings together two high-use drugs, applicable disease states, affected population, and number of patients/hospital and lists the appropriate LTA test(s). Clearly, the entire universe of these patients is not going to be tested. The point is that the patient pool needing these basic tests is over 700 times larger than the inherited platelet dysfunction group. These patients would require periodic testing over an extended time period. This is where our focus should be. Improving the adherence rate by routine monitoring can contribute significantly to reducing the mortality rate in these patient populations.
• SPA: Spontaneous Platelet Aggregation: saline is used in place of an agonist. Patient baseline. An SPA result greater than 7.5% indicates hyperactive platelets.18
• Acute Care Hospital (3,977/AHA); (39% of hospitals have cath labs: 1551)
• Each year, 15,000 people die and 100,000 people are hospitalized as the result of aspirin and other NSAIDs
REFERENCES
- MedicineNet.com. Definition of platelet. www.medicinenet.com/script/artasp?articlekey=4941.
- Gresele, P, Harrison, P, Bury, L, et al. (on behalf on the ISTH Platelet Physiology SCC). Diagnosis of suspected inherited platelet function disorders: results of a world wide survey. J Thromb Haemost. 2014;12(9):1562-1569.
- The Division of Blood Disorders, Registry for Bleeding Disorders Surveillance, Community Counts, The HTC Population Profile. The National Center for Birth Defects and Developmental Disabilities. Centers for Disease Control and Prevention. March 30, 2016 update.
- Cognasse F, Garraud, CF, Pozetto B, Laradi, S, Hamzeh-Cognasse, H. How can non-nucleated platelets be so smart? J Thromb Haemost. 2016;14(4):794-796.
- Li C, Li J, Li Y, et al. Crosstalk between platelets and the immune system: old systems with new discoveries. Advances in Hematology. Volume 2012 (2012). Article ID 3384685, 14 pages. http://dx.doi.org/10.1155/2012/384685.
- Krumholz, HM. Examining an ad for a platelet inhibition test. CardioExchange Archive NEJM. Feb 27, 2011.
- Schaef-Johns, G. Clopidogrel platelet function tests: caveats and controversies. Mayo Hot Topics, Dec. 2011.
- Futrell, K. The laboratory’s contribution to advanced medical analytics. MLO. 2016;48(2):30-34.
- Hugtenburg, JG, Timmers, L, Elders, PJ, Verloet M, van Dijk L. Definitions, variants and causes of non-adherence with medication: a challenge for tailored interventions. Patient Preference and Adherence. 2013;7:675 – 682. Doi:10.2147/PAA.S29549.
- Heneghan, CJ, Garcia-Alamino, JM,Spencer, EA, et. al. Self-monitoring and Self-management of oral anticoagulation. Cochrane Database. Rev. 2016 Jul 5;7: CD003839. Doi: 10:0.1002/14651858.CD003839.pub3.
- Elwyn G, Edwards A, Britten N, “Doing prescribing”: how doctors can be more effective. BMJ. 2003; 327(7419): 864-867.
- Mozaffarian, D, Benjamin, EJ, Amett, DK, et.al;. Heart Disease, Stroke and Research Statistics at a Glance-2016 Update: A Report from the American Heart Association, doi: 10.1161/CIR.0000000000000350.
- Agency for Healthcare Research and Quality. Weighted national estimates. HCUP National Inpatient Sample [online]. 2012. [cited 2015 Feb 9]. Available from: http://hcupnet.ahrq.gov/HCUPnet.jsp.
- Marss, SP. Optimizing the diabetic formulary: beyond aspirin and insulin. J Amer Coll Cardiol. 2002;40(4):652–661.
- LeFevre, ML(on behalf of the U.S. Preventive Services Task Force). Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2014;161:819-826.
- SEER Program. Surveillance Research Program. Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD. 2015.
- Langabeer, JR, Henry, TD, Kereiakes, DJ, et. al. Growth in Percutaneous Coronary Intervention Capacity Relative to Population and Disease Presence. J Am Heart Assoc. 2013;Oct 28;2(6):e000370. doi: 10.1161/JAHA.113.000370.
- Wu, KK, Barnes, RW, Hoak, JC. Platelet hyper reactivity in idiopathic, recurrent deep vein thrombosis, Circulation. 1976;53(4)687-690.
William M. Trolio, BS, MT, CLT, MBA, FBA, serves as Vice President and Chief Science Officer for Pennsylvania-based Bio/Data Corporation.