Rapid molecular platform
Alere i is a rapid molecular platform for the qualitative detection of infectious diseases; it is currently available and CLIA-waived for Strep A and Influenza A & B. The Alere i system is ideal for streamlining respiratory testing in the laboratory. Features include molecular results in minutes; intuitive, user-friendly design; a sample amplification process; small footprint; and connectivity to the Alere RALS system. On August 19, 2016, Alere announced that the Alere i RSV (respiratory syncytial virus) test received 510(k) marketing clearance from the FDA for the detection of RSV infection in children and adults. It is not currently available in any markets, but is expected to make its debut this fall. Alere
Droplet generator and reader
Medical practitioners in Europe can use Bio-Rad’s CE-IVD marked QX200 Droplet Digital PCR (ddPCR) System, IVD for highly accurate diagnostic detection and quantification of nucleic acids, aiding clinical decision-making in the treatment of diseases ranging from cancer to transplant rejection and viral infection. The system includes a QX200 Droplet Generator, QX200 Droplet Reader, and QuantaSoft software. The droplet generator and reader are CE-IVD marked for use in the European Union for in vitro diagnostics and are available for use in the clinical laboratory. Bio-Rad
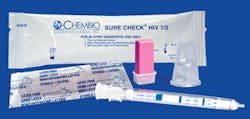
Self-contained collection and test device
Chembio Diagnostic Systems, Inc., is now marketing and selling its SURE CHECK HIV 1/2 Assay, which was previously marketed in the U.S. by Alere, Inc., as Clearview COMPLETE HIV 1/2 Assay, Part #92111. This change is in name only; the product has not undergone any changes in format or formulation and does not require re-validation. This product continues to be manufactured exclusively at Chembio’s FDA-registered, cGMP compliant and ISO 13485 certified facility in Medford, NY. SURE CHECK is an easy-to-use, self-contained collection and test device; is FDA-approved/CLIA waived (for fingerstick and whole blood); and requires only 2.5 µL of fingerstick or venous whole blood, serum, or plasma. Chembio
Hemostasis testing system
ACL AcuStar Hemostasis Testing System is a fully automated, high sensitivity chemiluminescent system designed for Hemostasis specialty and routine testing. HemosIL assays improve accuracy, range, sensitivity and specificity with the simplicity of ready-to-use reagent cartridges. High-performance chemiluminescent HemosIL assays include: D-Dimer, HIT-IgG(PF4-H)*, aCL IgG, aCL IgM, aB2GPI IgG, aB2GPI IgM, VWF:RCo*, VWF:Ag*, and VWF:CB*. It features self-contained, ready-to-use reagent cartridges, stable up to six weeks onboard; is available 24 hours/day, 7 days/week; features assay calibration on lot change only and random access: no batching required. *Not available in all countries. Not currently 510(k) cleared. Instrumentation Laboratory
Clinical chemistry ensemble
MedTest, exclusive U.S. distributor of the Mindray 480 Chemistry Analyzer, manufactures and distributes reagents, calibrators, and quality control products for the general chemistry and drugs of abuse testing markets. MedTest provides customized installation, on-site operator training, technical phone support, and on-site support if needed. The 480 Analyzer produces 400 photometric results per hour and 240 ISE tests per hour with an overall throughput of 560 tests per hour. Advanced features of the BS-480 analyzer include Auto-Start-Up, QC, Rerun, Pre-dilution, Post-dilution, ISE Calibration, Probe Cleaning, Reagent Blank Checks, and Probe Collision Recovery; which provides the laboratory with smooth operational and enhanced workflow efficiencies. MedTest
Mycoplasma direct test
The newly FDA-cleared illumigene Mycoplasma Direct test provides highly sensitive and specific results from day one of symptoms using throat swabs and a simple procedure that takes less than two minutes of hands-on time. The illumigene molecular platform requires no capital equipment expense or service contracts. The platform’s test menu also includes molecular assays for C. difficile, Group A Streptococcus, Group B Streptococcus, HSV 1&2, and pertussis in the United States, as well as chlamydia, gonorrhea, and malaria outside of the U.S. Meridian BioScience
Glucose hospital meter system
StatStrip Glucose Xpress2, StatStrip Glucose, and StatStrip Glucose Xpress are three hospital glucose meters that have been cleared by the FDA and proven to be safe and effective for use throughout all hospital and professional healthcare settings, including with critically ill patients. Use of other strip-based glucose meters with critically ill patients is considered “off-label” by the FDA and Centers for Medicare and Medicaid Services. StatStrip Glucose Xpress2 utilizes the same glucose measurement technology as StatStrip Glucose and StatStrip Glucose Xpress. StatStrip Glucose technology has been studied extensively, has been proven to be free of clinically significant interferences, and demonstrates excellent agreement with central laboratory reference methods. Nova BioMedical
Chemistry assay for procalcitonin
Roche has received 510(k) clearance for its Elecsys BRAHMS PCT (procalcitonin) assay as a dedicated testing solution for people with severe sepsis or septic shock. With this clearance, Roche provides a fully integrated solution for sepsis risk assessment and management. PCT is a sepsis-specific biomarker associated with bacterial infection, and PCT levels in blood can aid clinicians in assessing the risk of sepsis as well as managing the disease when present. The Elecsys BRAHMS PCT assay can aid in assessing the risk of critically ill patients to progress from severe sepsis to septic shock and help determine the 28-day mortality risk in sepsis patients. Roche
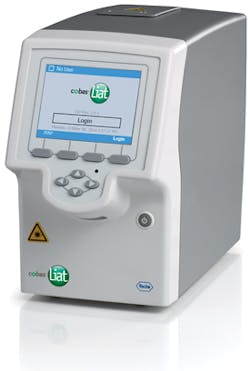
Flu and RSV test
Roche has announced that the FDA has granted 510(k) clearance and CLIA waiver for the cobas Influenza A & B and RSV test for use on the cobas Liat System. Roche is extending the value of highly accurate CLIA-waived molecular testing beyond flu A/B and strep A to include respiratory syncytial virus (RSV), a cause of more than 80 percent of acute lower respiratory tract infections in infants under one year of age. The cobas Influenza A & B and RSV test is the third assay on the cobas Liat System to receive CLIA waiver, following the cobas Strep A and cobas Influenza A & B tests, which received CLIA waiver in May and September 2015, respectively. The cobas Liat Analyzer and all three assays are FDA cleared and CLIA waived. Roche
Fully automated coagulation analyzer
The Sysmex CS-2500 System from Siemens Healthineers, a fully automated coagulation analyzer, offers mid-volume and multisite hemostasis labs smartly designed technologies—including PSI technology—to improve sample management, increase efficiency, and streamline lab workflow. The system offers a wide spectrum of testing methodologies and sophisticated software to simplify lab operations, and provides an uninterrupted workflow delivered in a compact, affordable footprint. Additionally, it offers lab-to-lab consistency for multisite patient monitoring, with sample result traceability for in-depth audit capabilities and sophisticated cap-piercing technology. Compact yet powerful, the Sysmex CS-2500 is designed to reduce costs, optimize workflow, and maximize operating efficiency. Siemens
Immunoassay system
The ADVIA Centaur XPT Immunoassay System from Siemens Healthineers is engineered for continuous operation, seamless connectivity, and timely, accurate results. Designed with one of the most advanced software packages on the market, the ADVIA Centaur XPT System is among the highest-throughput systems available. The system delivers the results that clinicians depend on for accurate diagnoses and better patient care, predictably and consistently. Siemens Healthineers unites innovative workflow solutions with clinical excellence in the ADVIA Centaur XPT System, leading to greater laboratory productivity to stay ahead of increasing workload demands. Siemens
Saliva assays
DRG Optimized Saliva ELISA Assays are easy-to-use, non-invasive sampling test kits designed for the measurement of the free (unbound) fraction of steroids. These tests allow for simple, patient-friendly measurement of hormone profiles. They are also a reliable measurement of steroids in veterinary saliva samples without further pre-treatment. DRG Optimized Saliva ELISAs include DHEA, Cortisol, Estradiol, Testosterone, and Progesterone. Features include: FDA approved; increased stability and linearity; ready-to-use reagents and user-friendly assays; non-invasive, easy sampling, particularly for babies and children; higher diagnostic relevance, with reproducible and reliable results, compared to serum analytics; and excellent correlation to mass spectrometry. DRG International
About the Author
Sign up for our eNewsletters
Get the latest news and updates