Urgency drives new techniques
The epidemic of prescription opioid abuse has been worsening for decades in the United States, but it has broken through to the public consciousness only in the past year or so. People have been sensitized to the problem by the deaths of celebrities from the misuse of opioid painkillers—most recently, of course, the celebrated musician Prince. The misuse of these drugs has also been discussed in the current presidential campaign, and federal and state efforts to combat it have frequently been in the news. The public attention is overdue; according to the Centers for Disease Control and Prevention, more than 18,000 deaths related to opioid overdose occurred in 2014.1
To combat this abuse crisis, certain states have legally mandated that healthcare providers require tests for patients receiving pain management drugs in an attempt to detect opioid misuse. This is not only to protect the patients by screening for overuse, but also to detect opioid levels below the dosage level, which could indicate that patients are reselling their drug refills. Because of the advent of these physician monitoring programs, laboratories must be able to accurately detect low levels of these drugs, in order to ultimately determine whether patients with chronic pain are abusing prescription opioids.
Screening options
Laboratories have a diverse choice of sample matrices to screen for opioid levels, but they ultimately need to choose the option that will maximize workflow and keep costs low. Whole blood is a unique matrix because it enables the analysis of the drug compounds in parent rather than metabolized form. However, it requires an invasive collection technique and can be challenging to pretreat due to the complexity. Oral fluid is a sample matrix that has had a lot of research focus recently. Although easy to collect, the collection devices used in oral fluid analysis can introduce their own unique challenges due to the presence of surfactants.2 Urine is a dependable sample matrix that doesn’t require the lengthy pretreatment steps that are necessary with whole blood, or the use of a collection device, which is required with oral fluid. It does, however, require a hydrolysis step to calculate correct concentrations of drug compounds. Due to the accuracy and precision associated with urine analysis and the developed methods, it’s widely considered to be the preferred sample matrix choice. It is both simple and inexpensive to perform opioid drug testing on urine.
ELISA vs. LC/MS
Laboratories running urine drug tests have traditionally used enzyme-linked immunosorbent assays (ELISA), but a case can be made that this testing approach is outdated and can be unreliable. For example, false negatives can alter the dependability of results, and the inability to accurately quantify drug concentrations leads to unreliable analysis.3 If ELISA tests are positive, the patient samples still need to be run on a mass spectrometer (MS) to quantify the concentration of drugs present. The time it takes to run an ELISA is comparable to other tests, but this method requires a more hands-on approach for laboratory technicians as well as separate tests for each drug analyte. It has been argued that liquid chromatography with mass spectrometry analysis (LC/MS) offers an efficient and easy way to separate and quantify a large class of opioid compounds. Many labs are moving toward testing with LC/MS first as advancements make it easier to run the tests and method development becomes simpler. They prefer chromatography over ELISA for the testing of many opioid drug compounds because they think that it meets their needs for increased accuracy, improved workflow efficiency, and reduced analysis time.
The metabolism of drugs must be understood in order to analyze urine by LC/MS. Before drugs exit the body through urine excretion, they are tagged with a glucuronic acid, which helps to change the polarity of the drug compound and aids in the absorption into the kidneys. Before chromatography analysis can occur, the glucuronide must be cleaved through hydrolysis. This can be done in two ways: acid hydrolysis and enzymatic hydrolysis. Some labs prefer enzymatic hydrolysis because it cleaves the bond without introducing harsh solvents into the sample or altering peaks.
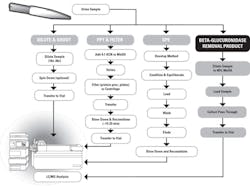
In enzymatic hydrolysis, ß-glucuronidase is introduced to cleave the glucuronide bond on the drug metabolite, resulting in free drug compounds.4,5 While this is effective, ß-glucuronidase can precipitate out in the LC column during the run, which can negatively affect the column’s selectivity and lifetime and can result in buildup in the MS. Analysts employ a variety of methods to prepare hydrolyzed urine samples for LC/MS analysis including “dilute-and-shoot,” protein precipitation, and solid phase extraction (SPE). The workflows associated with each technique are outlined in Figure 1.
“Dilute-and-shoot” vs. ß-glucuronidase removal
Most analysts in toxicology and clinical laboratories who utilize chromatography for urine analysis employ the “dilute-and-shoot” method as their sample preparation technique. They take the samples with residual ß-glucuronidase enzyme and “dilute” between 10 and 30 times the original volume. The diluted sample is then injected onto the LC column. However, the large dilution factor reduces the concentration of proteins/enzymes in the sample along with the concentration of analytes, which can result in poor sensitivity and can amplify any ion suppression that may be occurring in the analysis.2
Another deficiency of the “dilute-and-shoot” method is that it compromises the LC column. One way to help alleviate the enzymatic buildup during a “dilute-and-shoot” procedure is to centrifuge the sample to pellet the enzyme and collect the supernatant, but this does not completely remove ß-glucuronidase from the matrix (Figure 2).
One new ß-glucuronidase removal method is a sample preparation technique that removes ß-glucuronidase after enzymatic hydrolysis. The sorbent eliminates the ß-glucuronidase from the sample but does not interact with opioid analytes. While the technique requires rougly the same amount of time as a “dilute-and-shoot” procedure, about one minute, the sensitivity of the analytes is increased significantly. In one study, the sensitivity of THC-COOH was compared using the two preparation methods, and the new technique showed more than three times the sensitivity.
Due to the rapid demand for results, lab leaders have to evaluate methods for drug testing and choose the most efficient techniques to analyze samples. Sample matrices, types of analysis modes, and sample preparation techniques need to be considered when making this critical decision. Laboratories cannot afford any mistakes or lack of sensitivity when testing patients for opioid abuse. Inaccurate results could negatively affect the quality of life for patients using prescribed pain management drugs. Utilizing the most beneficial techniques for the laboratory helps to guarantee that patients who are required to undergo drug testing for their medications will not only get rapid answers but also accurate results as well.
REFERENCES
- National Vital Statistics System, Mortality File. Number and age-adjusted rates of drug-poisoning deaths involving opioid analgesics and heroin: United States, 2000–2014. Center of Disease and Control/ National Center for Health Statistics. 2015. http://www.cdc.gov/nchs/data/health_policy/AADR_drug_poisoning_involving_OA_Heroin_US_2000-2014.pdf.
- Edinboro LE, Backer RC, Poklis A. Direct analysis of opiates in urine by liquid chromatography-tandem mass spectrometry. Journal of Analytical Toxicology. 2005;29(7): 704-710.
- Allen K. Screening for drugs of abuse: which matrix, oral fluid or urine? Annals of Clinical Biochemistry. 2011;48(6):531-541.
- Lynch K, Wu A Yang, H. Development and validation of a novel LC-MS/MS opioid confirmation assay: evaluation of ß-glucuronidase enzymes and sample cleanup methods. Journal of Analytical Toxicology. 2016;40:323–329.
- Wang P, Stone JA, Chen KH, Gross SF, Haller CA, Wu AH. Incomplete recovery of prescription opioids in urine using enzymatic hydrolysis of glucuronide metabolites. Journal of Analytical Toxicology. 2006;30(8):570-575.
Jenny Cybulski graduated from Vanguard University with a BS in biology and a minor in chemistry. She now serves as a Product Communications Manager for Torrance, California-based Phenomenex, Inc.