The laboratory of the future moves outside the walls
The clinical laboratory world has undergone significant changes in technology, personnel training, and regulatory mandates over the past several decades that have had an impact on how patient care is provided. Laboratory testing has been recognized as an integral part in the diagnosis and treatment of diseases. Further, due to changes in payment reimbursements, strategies in managing a clinical laboratory have shifted, making laboratory testing a commodity. Yet, as has been well documented, 70 percent of medical decision-making is based on diagnostic laboratory testing, but testing accounts for only 2.3 percent of total healthcare expenditures.1
In addition, there has been a tremendous shift in how and where patients want to receive their healthcare and related information, thus placing a greater demand on physicians, nurses, and other healthcare practitioners to provide timely and accurate patient-focused services. Point-of-care testing, telehealth services, and direct access testing are three areas that have, in part, contributed to this ever-expanding desire for easy access, thus allowing individuals to have greater input in their healthcare decisions. Studies indicate that better patient care is provided when patients and their healthcare providers have easy access to clinical information.2
Point-of-care testing
Point-of-care testing (POCT), or near-patient testing, was introduced several decades ago and has, over time, been incorporated into the daily procedures found in many emergency departments, physician offices, nursing homes, neighborhood clinics, drugstores, and even in the home. The intent of POCT is to offer a way to facilitate patient treatment by targeting healthcare interventions in a more timely and convenient manner.3
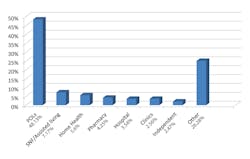
While less than four percent of CLIA-certified laboratories are within hospital settings, they perform the majority of laboratory tests (Figure 1).1 An estimated 12.8 billion laboratory tests are performed each year in the U.S.4 with approximately one-third of these tests involving POCT.5 POCT is largely based on CLIA-waived testing, which has expanded over the past few years, making it a significant testing modality within hospitals and other venues by working in concert with central laboratories (Table 1)24.
For example, in a survey of 317 U.S. hospitals, 100 percent of them were using POCT for glucose monitoring, 87 percent for coagulation monitoring, and 77 percent for blood gas/electrolytes monitoring—while 32 percent incorporated POCT for cardiac markers and 20 percent for HbA1c.6 In another study, 23 percent of hospitals had used six or more POCT instruments in 2011 compared to only five percent in 2007.7
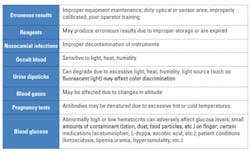
Concerns have arisen regarding the accuracy of POCT results, especially when proper quality assurance protocols are not followed. Table 2 lists some common areas that have been identified as sources for potential error.5,8,9 Yet POCT, when managed correctly, can provide accurate and timely diagnostic information that can improve patient care, decrease costs, and make better use of limited personnel resources.
Because of the relative ease of use and its portability, POCT can be done almost anywhere by anyone. Training is key to the proper use and maintenance of equipment used; so is implementing correct quality control procedures. Any deviation in protocol procedures, especially when multiple users are involved, can result in erroneous results. Also, use of multiple brands of POCT equipment can contribute to variable results; thus the need to standardize to a single model. Other concerns involve environmental factors of excessive heat, cold, or humidity due to improper shipping or storage conditions, causing reagent damage.
Confusion may also occur when comparing POCT results with those obtained from the central laboratory, as differences may occur due to variability in methodology. While there are many factors that lead to inaccurate POCT results, proper training, equipment maintenance, identifying unique patient conditions, and strictly adhering to POCT policies and procedures will minimize potential errors.
Telemedicine/telepathology
A number of healthcare areas have embraced telemedicine as an option to provide services remotely to a larger community base, especially in rural areas and in healthcare facilities that may not have access to certain healthcare specialists. Within the last few years, computer technology has improved to the level of meeting the needs of usable telepathology opportunities. High-definition monitors and extremely large storage capacities enable the transfer of image-rich pathology information that can be transmitted across the country and beyond.
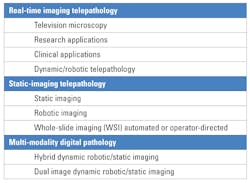
There are a number of imaging formats used in telepathology (Table 3).10,11 Static image technology has been around for a number of years but is limited to a few screen shots preselected by the sending facility. A video imaging system allows for real time, direct connectivity between two workstations with the sending facility controlling the microscopic images to be transmitted. Whereas, a robotic system gives a consulting pathologist the opportunity to directly manipulate a microscope from a distance in real time, thus allowing for expert review/interpretation of pathology slides. A fourth type of system is a whole slide imaging (WSI) system, which provides the option of scanning an entire slide, digitizing it, and transmitting a single file electronically via cloud technology.
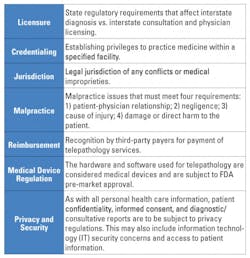
Telepathology as part of pathology services has made some inroads in the past few years, though it is still not extensively used in the United States. It has been more readily accepted in other countries as a way to obtain second opinions and conduct pathology slide reviews and frozen section evaluations, especially in underserved areas lacking certain types of expertise. Improved storage capacities and adequate transmission bandwidths are paving the way for the expansion of telepathology in the U.S., yet some concerns remain (Table 4).25
The second generation of “Web 2.0” offers even more support for telepathology.12 Web 2.0 offers a more robust interactive opportunity for pathologists to engage in editing, creating content, and commenting on shared cases. This higher level of mutual participation can offer greater diagnostic, consultative, and educational experiences in a timely and widespread manner. User-generated pathology content can be shared in a virtual community where information can be discussed in conjunction with other tele-diagnostic modalities—including x-rays, CT images, and ultrasound images—to provide overall better patient care.
Direct access testing
The gateway to proving direct access testing (DAT) began as early as the 1950s, when over-the-counter (OTC) test kits became available for measuring urine glucose and ketones in an effort to provide direct and timely access to a diabetic patient with potential health concerns.13,14 Web-savvy health consumers, who can seek out volumes of medical information and are generally better educated, want access to their own healthcare information in order to track health progress and treatment, and to advocate for themselves with their healthcare provider.
Further, maintaining a healthy lifestyle has been embraced by many and is often supported through employer-sponsored workplace wellness programs, such as smoking cessation, gym memberships, stress-control, weight loss, and nutritional offerings, especially in companies with greater than 200 employees.15 This has stimulated public demands to know what is in the products we consume, such as saturated fat content, sugar levels, milligrams of sodium, whether products are gluten-free, and whether they have been organically raised, or are vitamin-enriched. This “wellness revolution” has had an impact on the delivery of healthcare and laboratory services.16
The move to electronic health records (EHR) has also increased opportunities to easily share health information through secure web portals. Wireless technology through mobile apps and wearable devices can track and monitor basic health functions, providing additional ways to share health data. Subsequently, access to laboratory testing results has become a driving force behind the desire for the lay public to gain greater control of their healthcare information and not rely solely on their healthcare provider.
As of 2014, the Department of Health and Human Services (HHS) issued its final rule amending CLIA’88 and HIPAA privacy rules to allow patients to obtain their laboratory results directly from any CLIA-certified laboratory.4,17 Some states require the healthcare provider to send the results. Laboratories must release this information within 30 days of the patient’s request. The estimated cost for administering this program is $59 million over the first five years, which in part would be compensated for by charging patients nominal and reasonable fees for hard copy/mailing/labor costs. Implementation has been slow, with less than one-third of laboratories sharing direct access to clinical laboratory test results with patients, though that number is expected to grow quickly.2
More recently, consumer-directed laboratory test ordering has provided another opportunity for greater control over one’s own healthcare. As of this writing, 37 states and the District of Columbia allow for individuals to order their own laboratory tests without a healthcare provider’s input.18 This opportunity has not gone unrecognized by retail outlets, such as Walmart, Sam’s Club, Safeway, Costco, Walgreens, Concentra, and Whole Foods, which have paved the way to offering one-stop healthcare services including eye clinics, blood pressure testing, flu shots, and glucose and cholesterol POCT. Rite Aid Pharmacy has taken this a step further by introducing telemedicine kiosks in a limited number of stores to address minor health issues. These completely enclosed “health centers” offer basic services by connecting to a healthcare provider telephonically.19, 11
Conclusions
With all this external technology available, it is not surprising that laboratory testing has extended beyond the traditional laboratory footprint that encompasses large pieces of testing equipment and highly trained personnel. Extra-laboratory testing has become a growing business, with major opportunities for hospital laboratory leaders and other enterprising health professionals to work together to improve patient access to healthcare.
Continuing advances in testing technology, miniaturization of equipment, improved connectivity, and overall easier access to healthcare services and information are on the rise. New concepts include “lab-in-a-briefcase,” where diagnostic testing using a microfluidic ELISA platform can be performed and transported easily to any location in the world.20,21 Another evolving technology is “lab-on-a-chip” (LoC), which promises a wide range of laboratory analytics using a combination of nanotechnology, biotechnology, and microelectronics. A single drop of blood can be placed on a chip that may be used in the analysis of genetic material and specific analytes, offering more complex testing then what POCT currently provides.22
Smartphones already provide some basic health-monitoring functions. Using LoC technology in combination with smartphones is on the horizon.23 The synergy between these two technologies will use the advanced properties of communication, social networking, computation, and imaging in ways that will potentially provide quick and simple diagnostic information.
Laboratory services are now being offered beyond the traditional laboratory walls, using non-traditional methodologies in non-traditional locations. Continuing improvements in technology will create an even greater demand for extra-laboratory testing options, empowering individuals by enabling them to play a more active role in managing their healthcare.
REFERENCES
- Adva MeDx. A policy primer on diagnostics. June 2011. http://advameddx.org/download/files/sections/Policy/Innovation/AdvaMedDx-Policy-Primer-on-Diagnostics-June-2011.pdf.
- Swain M, Patel V. Patient access to test results among clinical laboratories. The Office of the National Coordinator for Health Information Technology. ONC Brief #13, Feb 2014. https://www.healthit.gov/sites/default/files/onc-data-brief-13-labsurveydatabrief.pdf.
- Kurec AS. Trends in point-of-care testing. Health Management. 14(4):76-80, October 6, 2014. http://healthmanagement.org/s/trends-in-point-of-care-testing.
- Clinical Laboratory Improvement Amendments (CLIA). https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Downloads/factype.pdf.
- Nichols JH. Point of care testing. Clin Lab Med. 2007;27:893-908.
- Winter MC. Marketing trends in point-of-care testing 2007-2008. Point of Care: The J Near-patient Testing & Technol. 2010; 9(1):12-20.
- Malone B. Spotlight on point-of-care testing. Clin Lab News.Oct 1, 2012. https://www.aacc.org/publications/cln/articles/2012/october/point-of-care-testing.
- Ginsberg BH. Factors affecting blood glucose monitoring: Sources of errors in measurement. J Diabet Sci Technol 2009; 3(4):903-913.
- Johnson S, Cushion M, Bond S, et al. Comparison of analytical sensitivity and women’s home pregnancy tests. Clin Chem Lab Med. 2015; 53(3):3911-402.
- Weinstein RS, Graham AR, Lian F et al. Reconciliation of diverse telepathology system designs. Historic issues and implications for emerging markets and new applications. APMIS. 2012;120:256-275.
- Pantanowitz L. American Telemedicine Association (ATA): New Telepathology Guidelines. Pathology Informatics Summit. 2014. http://www.pathologyinformatics.com/sites/default/files/archives/2014/Day/20140514%201645%20-%20American%20Telemedicine%20Association%20New%20Telepathology%20Guidelines.pdf.
- LeFevre P. Digital pathology. Pathology innovators use Web 2.0 to boost productivity and create clinical value. Dark Daily: Whitepaper. (2009) http://www.darkdaily.com/white-papers/digital-pathology-white-paper-pathology-innovators-use-web-2-0-to-boost-productivity-and-create-clinical-value#axzz3tTWxoE46.
- American Society for Clinical Laboratory Science. Consumer access to laboratory testing and information classification: A position paper. July 2012. http://www.ascls.org/position-papers/177-direct-access-testing.
- Lab Testing Matters. Direct access to test results. July 2, 2015 http://www.labtestingmatters.org/direct-access-to-test-results/.
- Robert Wood Johnson Foundation. Workplace Wellness Programs. Healthcare Policy Brief. May 16, 2013. http://healthaffairs.org/healthpolicybriefs/brief_pdfs/healthpolicybrief_93.pdf.
- Test Country.com. Direct access testing. http://www.testcountry.com/content/direct-access-testing.html/.
- Department of Health and Human Services. Billing code 4120-01-P. http://www.hpm.com/pdf/blog/CLIA%20Program%20and%20HIPAA%20Privacy%20Rule.pdf/.
- AACC Report: Direct-to-consumer test results should be more patient-friendly. Lab Medica International 2015;32(6):1.
- Kirk P. Rite Aid’s New Telemedicine Kiosks in Select Ohio Pharmacies Allow Customers to Consult with Physicians for a Nominal Fee. Dark Daily: April 3, 2015. http://www.darkdaily.com/rite-aids-new-telemedicine-kiosks-in-select-ohio-pharmacies-allow-customers-to-consult-with-physicians-for-a-nominal-fee-403#axzz3ol0CHznK.
- Barbosa AI, Castanheira AP. Edwards AD, et al. A lab-in-a-briefcase for rapid prostate specific antigen (PSA) screening from whole blood. Lab Chip 2014; 14:2918. http://pubs.rsc.org/en/content/articlelanding/2014/lc/c4lc00464g#!divAbstract.
- LabMedica Staff Writers. First lab-in-a-briefcase to boost global early diagnosis of cancer. LabMedica. Nov 18, 2015. http://www.labmedica.com/lab-technology/articles/294761397/first-lab-in-a-briefcase-to-boost-global-early-diagnosis-of-cancer.html.
- Whitesides G. The lab finally comes to the chip! Lab Chip. 2014; 14:3125. http://pubs.rsc.org/en/content/articlelanding/2014/lc/c4lc90072c/unauth#!divAbstract.
- Erickson D, O’Dell D, Jiang L, et al. Smartphone technology can be transformative to the deployment of lab-on-a-chip diagnostics. Lab Chip. 2014;14:3159. http://pubs.rsc.org/en/content/articlelanding/2014/lc/c4lc00142g/Unauth#!divAbstract.
- Washington State Department of Health. Waived tests and CPT codes. Revised 4/01/2015. http://www.doh.wa.gov/portals/1/Documents/Pubs/681018.pdf.
- Cornish TC, McClintock DS. Medicolegal and regulatory aspects of whole slide image-based telepathology. Diag Histopathol. 2014;20(12):475-481.
About the Author

Anthony Kurec, MS, MASCP, MLT, H(ASCP)DLM
is Clinical Associate Professor, Emeritus, at SUNY Upstate Medical University in Syracuse, NY. He is also a member of the MLO Editorial Advisory Board.