Clinical application of thromboelastometry in cardiovascular and thoracic surgery
Rotational thromboelastometry (ROTEM) was developed in the 1990s to address a number of technical challenges associated with the classical thromboelastography method. A more comprehensive review of thromboelastometry has been published in a previous issue of MLO1 and has been described by several others.2-4 This article focuses on the application of thromboelastometry in the clinical setting of cardiovascular and thoracic surgery (CVTS).
Coagulopathy: common in CVTS
Hemostasis imbalance in the patient population undergoing CVTS is quite common. In the pre-surgical environment, patients are often on a regimen of anti-platelet agents for the prevention of myocardial infarction and stent thrombosis. The antiplatelet effects of aspirin and clopidogrel are associated with an increased risk of bleeding and blood loss in cardiac surgery.5-9 Therefore, it is important to assess the therapeutic effect of these agents in patients being evaluated for surgical intervention.
Patients presenting with an acute condition requiring immediate surgical interventions also have the possibility of platelet inhibition due to a number of other drugs administered during percutaneous coronary intervention (PCI) for thrombosis prophylaxis or may be on long-term anticoagulation therapy such as with warfarin (Vitamin K antagonist). Drugs such as heparin (anti-Xa), abciximab (GP IIb/IIIa antagonists) and bivalirudin, a direct thrombin inhibitor (DTI), are often given as adjuvant therapy in the cardiac catheterization lab. Fibrinolytic agents such as tPA are also given to provide localized clot lysis in the coronary vasculature in order to clear nascent thrombus in the coronary arteries. These therapies occur in the pre-surgical environment and result in deliberate impairment of the pro-coagulant and fibrin polymerization hemostasis mechanisms.
Therefore, coagulopathic bleeding as a result of inhibiting clot initiation (via impairment of enzymatic factors), platelet function (via inhibiting platelet aggregation and inhibition of thrombin formation), and impaired fibrin-clot sustainability (due to induced clot lysis) can often accompany the patient to the intraoperative environment.
Factors originating in the intraoperative environment, such as hemodilution, fibrinogen and platelet consumption, heparin anticoagulation, hypothermia, and contact activation from the surfaces of the extracorporeal circuit used for cardiopulmonary support also contribute to common coagulopathic challenges. Hemodilution occurs primarily as a result of the initiation of cardiopulmonary bypass (CPB) due to replacing a portion of the patient’s blood volume with the priming solution (crystalloids and colloids) in the CPB circuit. Advances in techniques and technology, for example, the use of low volume circuits, have helped to minimize this effect in recent years, but it remains a primary cause of hemodilution.
In preparation for CPB support, high levels of heparin are administered for thrombosis prophylaxis. Mismanagement of heparin anticoagulation and subsequent reversal with protamine sulfate is a common contributor of coagulopathic bleeding in the perioperative setting.10-13 As well, previous exposure to heparin from prior interventional procedures may lead to the development of antibodies resulting in a heparin-induced thrombocytopenia (HIT). Unrecognized, HIT can result in a multitude of hemostatic derangements and requires alternate strategies of anticoagulation for thrombus prophylaxis during CVTS. Contact activation via the CPB circuit can contribute to consumptive coagulopathies and pathologic fibrinolysis,14 which can lead to excessive bleeding in the intra-operative and post-operative environments.
In the post-operative setting, increased chest tube output (CTO), along with a drop in hemoglobin and the associated physiological responses to increasing anemia, are the indicators for excessive blood loss. What is often unclear is whether this bleeding is due to conditions of a surgical or coagulopathic nature. Surgical bleeding may be due to a missing or loose suture along an anastomosis line or an un-ligated vessel. Coagulopathic bleeding can be due to the same factors that complicate bleeding in the intraoperative setting, for example, dilution of clotting factors, heparin rebound effect, and pathologic clot lysis. Also, abnormal basic conditions such as core temperature, Ca++ levels, hemoglobin, and acidosis can be contributing factors and should be considered when faced with excessive post-operative bleeding. Timely differentiation between surgical and coagulopathic bleeding is key to implementing the correct therapeutic intervention—surgical re-exploration or transfusion therapy to stop the bleeding.15
TEM-aided diagnosis
In addition to standard lab testing (SLT), viscoelastic testing (VET), as with rotational thromboelastometry (ROTEM) or thrombelastography (TEG), may be useful for the rapid assessment and identification of hemostatic imbalance that may contribute to active bleeding, the risk of bleeding, or the risk of thrombosis.16-18 Patients experiencing clinically significant bleeding in the perioperative setting may have enzymatic factor deficiencies, platelet dysfunction, and fibrin polymerization disorders that may be specifically identified rapidly with ROTEM or TEG. In non-actively bleeding patients, a level of hemostatic reserve may be assessed and considered when additional volume replacement may push the balance toward a hypo-coagulable state. ROTEM analysis has been shown to correlate well with and is predictive of CTO10,19 post-operatively in cardiac surgery. Conditions of hypercoagulability can also be assessed and identified for an increased risk of thrombosis.20,21
One VET system uses a panel approach to assess hemostasis and identify specific deficiencies. It can demonstrate impairment of clot initiation (due to enzymatic factor deficiency or drug effects), a slow clot propagation rate, weakened clot firmness (platelet or fibrinogen deficiency or dysfunction), and premature clot lysis leading to coagulopathic bleeding22 This is done using a citrated whole blood sample, and results are typically available in 15 minutes or less.23 Therefore, VET analysis may have some advantages over SLT for guiding therapeutic intervention to actively bleeding patients.
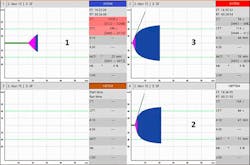
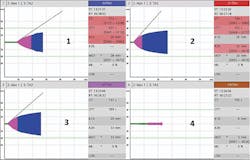
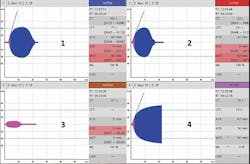
By comparing the clotting time parameter (CT) between INTEM and HEPTEM assays, the effect of heparin may be confirmed or ruled out as a contributor to bleeding (Figure 1). While on CPB support, the HEPTEM assays alone or the combination of EXTEM and FIBTEM may be useful to predict the hemostatic capacity after heparin reversal with protamine sulfate.16-18,24 Dilutional coagulopathy may be identified by looking at the clot formation time (CFT) and amplitude parameters (A10, A20, MCF) in the global hemostasis assays, i.e., INTEM, EXTEM and HEPTEM. Concurrent testing with the FIBTEM assay (which looks at the fibrin clot minus platelets), can aid with determining that any deficiency in clot firmness is primarily due to a fibrinogen, a platelet issue, or both (Figure 2). It has been shown that MCF EXTEM – MCF FIBTEM results in a reliable albeit indirect assessment of platelet contribution to the clot firmness with good correlation to platelet count.17 Premature clot lysis or hyperfibrinolysis can be identified by looking at the lysis parameter maximum lysis (ML) in the global ROTEM assays but is confirmed by the ML of the FIBTEM assay and/or the APTEM assay (which inhibits fibrinolysis in-vitro) to be true lysis versus a platelet-mediated clot retraction (Figure 3)
Figure 1. Heparin effect. Panel 1: (INTEM) exhibits a delay in clot initiation; Panel 2: (HEPTEM) assay contains heparinase and exhibits a correction in clot initiation confirming heparin effect; Panel 3: (EXTEM) exhibits a normal curve since it contains the heparin inhibitor polybrene.
Figure 2. Low clot firmness/hypofibrinogenemia. Panels 1 and 2 (INTEM & EXTEM) exhibit delayed clot initiation, prolonged clot propagation (CFT) and low clot firmness at A10; Panel 3 (APTEM) exhibits no improvement compared to EXTEM, indicating no hyperfibrinolysis; Panel 4 (FIBTEM) exhibits very low amplitude at A10, indicating severe hypofibrinogenemia. The delay in clot initiation in all assays is also likely due to low fibrinogen contribution rather than decreased thrombin generation. A recheck after fibrinogen substitution if bleeding persists is indicated.
Goal-directed therapy
Diagnostics without a protocol for therapeutic intervention will not stop bleeding. Successful implementation of thromboelastometry as part of a bleeding management program involves the development of treatment algorithms and testing protocols. Cardiac surgery protocols with assay-specific trigger values and treatment algorithms can be developed via review of current literature, industry supported education, and peer-provided assistance. There are several examples of ROTEM- and TEG-based treatment algorithms published in a variety of journals.15, 24-29 It is important to adapt or develop an algorthm that is easy to understand and works with the available therapeutic options. Algorithms also provide for a better chance that results between care providers are consistent, which may optimize transfusion strategies and patient results. Algorithms can help guide the transfusion (or avoidance) of allogeneic blood products (FFP, platelets or cryoprecipitate) or the off-label use of factor concentrates if permissible and validated for use by the facility.
Summary
VET with ROTEM and TEG have become more integrated into the practice guidelines of multiple medical associations with increasing levels of evidence and recommendation for their use to manage perioperative bleeding.30-31 VET, in conjunction with SLT, provides a clearer picture of why a patient bleeds and how to best resolve it. In the clinical setting of CVTS, ROTEM has been shown to reduce transfusion rates and reduce costs associated with transfusion products and transfusion-related adverse events while improving patient outcomes.15, 24-29 Incorporating VET into a hospital-wide transfusion restrictive strategy has been proven to be cost-effective and clinically beneficial15, 24-29 but requires a multi-level commitment for planning, education and implementation.
References
- Allen, T. Thromboelastometry: its method, application and benefits. MLO. 2014; 46(2):26-29. https://www.mlo-online.com/articles/201402/thromboelastometry-its-methodology-application-and-benefits.php. Accessed August 2, 2015.
- Tanaka KA, Bolliger D, Vadlamudi R, et al. Rotational thromboelastometry (ROTEM)-based coagulation management in cardiac surgery and major trauma. J Cardiothorac Vasc Anesth. 2012;26(6):1083-1093.
- Görlinger K, Jambo C, Hanke AA, et al. Perioperative coagulation management and control of platelet transfusion by point-of-care platelet function analysis. Transfus. Med. Hemother. 2007;34(6):396-411.
- Bolliger D; Seeberger MD; Tanaka KA, et al. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev. 2012;26(1):1-13.
- Hongo RH, Ley J, Dick SE, et al. The effect of clopidogrel in combination with aspirin when given before coronary artery bypass grafting. J Am Coll Cardiol. 2002;40(2):231-237.
- Yende S; Wunderink, RG. Effect of clopidogrel on bleeding after coronary artery bypass surgery. Critical Care Med 2001;29(12):2271-2275.
- Petricevic M, Biocina B, Boban M, et al. Bleeding risk assessment using whole blood impedance aggregometry and rotational thromboelastometry in patients following cardiac surgery. J Thromb Thrombolysis. 2013;36(4):514-526.
- Ranucci M, Baryshnikova E, Soro G, et al. Multiple electrode whole-blood aggregometry and bleeding in cardiac surgery patients receiving thienopyridines. Ann Thorac Surg. 2011;91(1):123-129.
- Corredor C, Wasowicz M, Karkouti K, et al. The role of point-of-care platelet function testing in predicting postoperative bleeding following cardiac surgery: a systematic review and meta-analysis. Anaesthesia. 2015;(6):715-731.
- Petricevic M, Biocina B, Milicic D, et al. Activated coagulation time vs intrinsically activated modified rotational thromboelastometry in assessment of hemostatic disturbances and blood loss after protamine administration in elective cardiac surgery: analysis from the clinical trial (NCT01281397). J Cardiothorac Surg. 2014;9(1):129.
- Koster A, Börgermann J, Gummert J, et al. Protamine overdose and its impact on coagulation, bleeding, and transfusions after cardiopulmonary bypass: results of a randomized double-blind controlled pilot study. Clin Appl Thromb Hemost. 2014; 20(3):290-295.
- Vonk AB, Veerhoek D; van den Brom CE, et al. Individualized heparin and protamine management improves rotational thromboelastometric parameters and postoperative hemostasis in valve surgery. J Cardiothorac Vasc Anesth. 2014;28(2):235-241.
- Ortmann E, Klein AA, Sharples LD, et al. Point-of-care assessment of hypothermia and protamine-induced platelet dysfunction with multiple electrode aggregometry (Multiplate) in patients undergoing cardiopulmonary bypass. Anesth Analg. 2013;116(3):533-540.
- Chandler WL, Velan T. Plasmin generation and D-dimer formation during cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2004;15(7):583-591.
- Weber CF, Görlinger K, Meininger, D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117(3):531-547.
- Gronchi F, Perret A, Ferrari E, et al. Validation of rotational thromboelastometry during cardiopulmonary bypass: A prospective, observational in-vivo study. Eur J Anaesthesiol. 2014;31(2):68-75.
- Olde Engberink RH, Kuiper GJ, Wetzels RJ, et al. Rapid and correct prediction of thrombocytopenia and hypofibrinogenemia with rotational thromboelastometry in cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28(2):210-216.
- Ortmann E, Rubino A, Altermimi B, et al. Validation of viscoelastic coagulation tests during cardiopulmonary bypass. J Thromb Haemost. 2015; 13(7):1207-1216.
- Reinhöfer M, Brauer M, Franke U, et al. The value of rotation thromboelastometry to monitor disturbed perioperative haemostasis and bleeding risk in patients with cardiopulmonary bypass. Blood Coagul. Fibrinolysis. 2008;19(3):212-219.
- Dimitrova-Karamfilova A; Patokova Y; Solarova T, et al. Rotation thromboelastography for assessment of hypercoagulation and thrombosis in patients with cardiovascular diseases. J Life Science. 2012;6:28-35.
- Hinker A, Feit J, Sladen, RN, et al. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care. 2014;18(5):549.
- Larsen OH, Fenger-Eriksen C, Christiansen, K, et al. Diagnostic performance and therapeutic consequence of thromboelastometry activated by kaolin versus a panel of specific reagents. Anesthesiology. 2011;115(2):294-302.
- Haas T, Spielmann N, Mauch J, et al. Reproducibility of thromboelastometry (ROTEM): Point-of-care versus hospital laboratory performance. Scand J Clin Lab Invest. 2012;72(4):313-317.
- Karkouti K, McCluskey SA, Callum J et al. Evaluation of a novel transfusion algorithm employing point-of-care coagulation assays in cardiac surgery: a retrospective cohort study with interrupted time-series analysis. Anesthesiology. 2015;122(3):560-570.
- Görlinger K, Shore-Lesserson L, Dirkmann D, et al. Management of hemorrhage in cardiothoracic surgery. J Cardiothorac Vasc Anesth. 2013;27(4 Suppl):S20-34.
- Anderson L, Quasim I, Soutar R, et al. An audit of red cell and blood product use after the institution of thromboelastometry in a cardiac intensive care unit. Transfus Med. 2006;16(1):31-39.
- Spalding GJ, Hartrumpf M; Sierig T, et al. Cost reduction of perioperative coagulation management in cardiac surgery: value of “bedside” thromboelastography (ROTEM). Eur Cardiothorac Surg. 2007;31(6):1052-1057.
- Pearse BL, Smith I, Faulke D, et al. Protocol guided bleeding management improves cardiac surgery patient outcomes. Vox Sang 2015 Apr 30. doi:10.1111/vox.12279. [Epub ahead of print]
- Nakayama Y, Nakajim Y, Tanaka KA, et al. Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after pediatric cardiac surgery. Br J Anaesth. 2015;114(1):91-102.
- American Society of Anesthesiologists: Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology 2015;122: 241-275.
- Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30(6):270-382.