According to the New England Journal of Medicine, an estimated one billion people worldwide are vitamin D deficient or insufficient.1 Experts believe at least half of adults over the age of 65 years have reduced bone density.2 Major causes are the lack of exposure to sunshine and an inadequate supply of vitamin D from foods. Physicians and patients are increasingly aware of vitamin D insufficiency, and labs are responding.
Why is vitamin D important?
Vitamin D is vital for the growth and health of bones because of its key role in regulating the absorption of calcium, phosphorus, and magnesium. Without vitamin D, only 10% to 15% of calcium is absorbed,1 which can cause soft and malformed bones, decreases in bone density (osteopenia), broken bones, and bone thinning (osteoporosis). In severe cases, bones are unable to repair themselves, a condition known as rickets in children and osteomalacia in adults. Vitamin D deficiency has also been associated with many non-bone related conditions, including autoimmune disorders, cardiovascular disease, and cancer,3 though these connections are not considered to be proven.
Why is vitamin D deficiency so common?
The “D” represents the two major forms of vitamin D, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Both forms can be gained from food sources and supplements; however, only vitamin D3 is synthesized when the skin is exposed to ultraviolet B radiation from the sun.
Small amounts of vitamin D2 can be found in fungi, while vitamin D3 can be found in egg yolks, salmon, sardines, tuna, and cod liver oil. Because of the difficulty in obtaining adequate vitamin D from food sources, vitamin D has been added to fortify certain dairy products, orange juice, infant formula, and cereals. Dietary supplements are available in the form of over-the-counter multivitamins and pills; oral and injectable prescriptions are also available.
The synthesis and metabolism of vitamin D is a complex process involving the skin, liver, intestines, and kidneys. The primary storage form of vitamin D resides in the liver. The intermediate form, total 25-hydroxyvitamin D (25[OH]D), is created in the liver and is the preferred biomarker of vitamin D status, which is the sum of 25(OH)D2 and 25(OH)D3 concentrations. The biologically active form, 1,25(OH)2D, is synthesized in the kidney and has direct actions on bone and muscle.
How much vitamin D is needed?
Although many individuals are considered vitamin D-insufficient, there is no consensus on optimal concentrations to gauge vitamin D status or recommended daily intake. The Endocrine Society recommends the following ranges:1
Deficiency (<20 ng/mL)The skin, the largest organ in the human body, has the greatest potential for vitamin D synthesis. However, vitamin D absorption varies among individuals based on latitude, elevation, skin color, age, time of exposure to sun, body mass index, cloud cover, use of sunscreen, month of year, and clothing. Thirty minutes of sun exposure can produce 20,000 IU of vitamin D, which does not lead to toxicity.4 The Institute of Medicine recommended daily intake is 200-600 IU/day depending on age, previous vitamin D blood levels, and what is causing the level to be low. Other experts state what without sun exposure, the recommended daily intake is 800-1000 IU/day.1 Toxicity can occur with supplements, but it is extremely rare.
Why should total 25(OH) vitamin D be measured?
There are several rationales for measuring 25(OH) vitamin D.
- 25(OH) vitamin D reflects the contributions from both de novo cutaneous synthesis and dietary intake, and this form is considered a circulating, ready store of vitamin D that can be activated on demand to 1,25(OH)2 vitamin D. As an analytical advantage, 25(OH)D circulates at about a 1,000-fold greater molar concentration compared to 1,25(OH)2D, making accurate measurement more easily accomplished.
- 25(OH) vitamin D circulates with a longer half-life in blood and is not affected by fluctuations in calcium or parathyroid hormone levels, simplifying its interpretation.
- 25(OH) vitamin D correlates best with clinical vitamin D status in patients.
- Because serum 25(OH) vitamin D will be a mixture of the D2 and D3 forms, both the vitamin D2 and vitamin D3 forms of vitamin D must be measured to accurately assess total 25(OH) vitamin D levels.
Available methods for measuring vitamin D include immunoassay and chromatographic. Immunoassays have been the mainstay of vitamin D testing, producing most of the history literature reports. Immunoassays are familiar to the routine clinical laboratory and can be added to the test menu of standard immunoassay systems. HPLC with tandem mass spectrometry detection (LC-MS/MS) provides robust and reliable vitamin D measurement, although system start-up cost and the need for highly trained operators can be issues for implementing this technology.
An important side note to vitamin D testing is that a recent U.S. Preventive Services Task Force (USPSTF) draft recommendation stated there is insufficient evidence that routine screening is effective. The USPSTF concluded that further studies are necessary to assess the balance of benefits and harms of screening the population for vitamin D deficiency.5
How are vitamin D assays standardized?
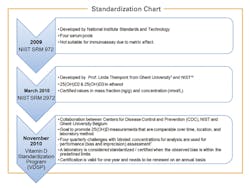
Standardization refers to the process of alignment with the “true” value. There is currently no World Health Organization (WHO) reference material available for calibrating vitamin D methods, but initial steps have been taken towards standardization (Figure 1), through the development of two internationally recognized “gold-standard” Reference Method Procedures (RMPs) available for vitamin D, one developed by the National Institute of Standards and Technology (NIST)6 and a second ID-LC-MS/MS method traceable to NIST SRM 2972, developed by Professor Linda Thienpont from Ghent University.7 Concentration values obtained using the NIST or the Ghent RMPs are considered to be the true concentrations.
Why is vitamin D technically challenging?
Vitamin D measurement is challenging for several reasons, including the already mentioned standardization issue, its highly lipophilic nature, its strong association with binding proteins, and the fact that the assay must recognize multiple forms of the molecule (D2 and D3) equally. It is important to note that not all commercially available vitamin D assays detect 25(OH)D2 and D3 in an equimolar fashion, with various methods under- or over-recovering D2 compared to D3. From the clinical point of view, this can produce confusing results in some patients (e.g., patients being monitored during high-dose vitamin D2 therapy).
The presence of different metabolites of vitamin D may also have the potential to interfere in immunoassays or LC-MS/MS methods. One form of vitamin D that deserves mention is an epimer of 25(OH)D at the hydroxyl group on carbon-3 of vitamin D. Generally, this metabolite affects quantification of 25(OH)D3 in most routine liquid chromatographic methods, and immunoassay can be affected to varying degrees. Although the function of the C3 epimer is unknown, it is considered to have a negligible effect on most routine assays for vitamin D.8
Like most steroid molecules in blood, vitamin D circulates bound to a carrier protein, vitamin D binding protein (VDBP). VDBP increases in pregnancy, oral contraceptive use, and hormone replacement therapy and decreases in renal disease, proteinuria, intensive care patients, and malnourished patients.9 To measure vitamin D, the analyte must be completely dissociated from VDBP. In chromatographic methods, this is accomplished during the extraction step, which separates the vitamin D from VDBP and other proteins in serum. Because the organic solvents used in chromatographic methods are not compatible with immunoassays, other methods of separation, such as proprietary chemicals, must be used. Increased or decreased levels of VDBP in individual patients may complicate vitamin D dissociation from VDBP and result in poor accuracy of immunoassays, making consistent separation of vitamin D from VDBP essential.
How does your assay measure up?
- Is the assay calibration traceable to the NIST reference material and/or the Ghent reference method procedure?
- Is vitamin D2 and D3 measured in an equimolar fashion?
- Does the assay formulation adequately separate vitamin D from its binding protein? Does the assay reliably measure vitamin D in cases of vitamin D binding protein excess, either pathologic or physiologic?
- Does the assay offer a reasonable analytical measuring range?
- Does the assay offer adequate precision without impacting clinical interpretation?
- Does the test system throughput adequately handle your laboratory workload?
High volume vitamin D testing is here to stay, at least for the time being. There are many options for vitamin D testing, and thoughtful consideration of your laboratory’s needs can help identify the right testing solution.
References
- Holick MF, Binkley N, Gordon C, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911‐1930.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporosis International. Published online April 27, 2010.
- Souberbielle JC. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmunity Reviews. 2010;9:709-715.
- Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Alternative Medicine Review. 2008;13:6-20.
- USPSTF Vitamin D Draft Recommendation Statement http://www.uspreventiveservicestaskforce.org/uspstf13/vitddefic/vitddeficdraftfact.pdf. Accessed September 22, 2014.
- Notice of vitamin D standardization program. A notice by the National Institutes of Health on 03/02/2011. http://www.federalregister.gov/articles/2011/03/02/2011-4603/noticeof-vitamin-d-standardization-program. Accessed September 22, 2014.
- Stepman HCM, Thienpont L, Uytfanghe K, et al. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography–tandem mass spectrometry. Clinical Chemistry. 2011;57(3):441–448.
- Keevil B. Does the presence of 3-epi-25OHD 3 affect the routine measurement of vitamin D using liquid chromatography tandem mass spectrometry? Clin Chem Lab Med. 2012;50(1):181–183.
- Heijboer AC. Accuracy of 6 routine 25-hydroxyvitamin D assays: Influence of vitamin D binding protein concentration. Clinical Chemistry. 2012;58(3):543–548.
- NIST SRM2972:25 Hydroxyvitamin D2 and D3 Calibration Solutions. SRM Spotlight. March 2010.