Every day, thousands of surgical procedures are performed in the United States. For each procedure, anesthesiologists and surgeons are faced with the question of whether or not to order blood products for potential intraoperative use. Orders may include blood typing and red cell antibody screening (T/S) or a type and crossmatch (T/C). Ordering blood products can be controversial. If products are ordered liberally, resources are stretched, increasing costs, creating a backlog of samples at the blood bank, and holding blood units for a single patient unnecessarily. On the other hand, if products are not ordered when needed, an unexpected hemorrhage in the operating room could be catastrophic. Appropriate preoperative blood ordering has the potential to both decrease costs and improve patient safety.
In 1976, Friedman1 created a Maximum Surgical Blood Order Schedule (MSBOS), which included blood ordering guidelines for common procedures. Since that time, many new procedures and surgical techniques have been introduced, requiring updated guidelines. Institutions often create an MSBOS, but this is usually based on the consensus of their surgeons and their blood bank. The ideal MSBOS should be procedure-specific, institution-specific, and based on objective blood use data. In the Department of Anesthesiology and Critical Care Medicine at Johns Hopkins, our team sought to create an MSBOS for our institution, as well as an algorithm for other institutions to create their own.2
Increasingly, anesthesiologists are abandoning paper charting for real time electronic data collection through an Anesthesia Information Management System (AIMS). An AIMS creates an accurate record of each surgical case, including vital signs, medications administered, surgical events, and even blood utilization data. Researchers can use these electronic data to study multiple parameters in an effort to improve care, including blood management. Our institution began using our AIMS in January 2010.
For our current study, we collected AIMS data for 27 months, from January 2010 through March 2012, including 53,526 adult surgical patients undergoing 1,632 types of procedures. (We excluded ophthalmic surgery patients who are never transfused.) The procedures were then grouped into 135 categories by anatomical operative site and surgical subspecialty. For each category, we analyzed the number of patients who had a preoperative T/S or T/C as well as the number of patients transfused with erythrocytes and the number of units transfused. The transfusion index (average number of erythrocyte units/patient) and median estimated blood loss were also recorded. We developed an additional parameter that we called “risk of major bleeding” and assigned each procedure category a “yes” or “no” after discussion with surgeons and consensus of our team. This parameter was largely based on the proximity to major blood vessels and the resultant potential for large intraoperative blood loss.
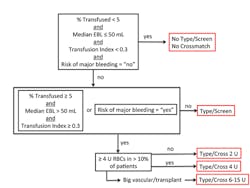
Based on these parameters and previously proposed criteria, we developed an algorithm to determine the appropriate blood order for each procedure category (Figure 1). There were three blood orders resulting from our algorithm: “no T/S or T/C,” “T/S,” or “T/C.” The “T/C” category is further broken down into a crossmatch for either 2 U (units), 4 U, or 6-15 U, based on the average number of units transfused for the procedure category.
Our 135 procedure categories were assigned to one of these five blood order groups. Fifty-two percent of patients had procedures that met the criteria for “no T/S or T/C”; however, 33% of these patients had a preoperative T/S and 10% had a T/C; only 1% of these 27,825 patients received a transfusion. Nine percent of patients met criteria for the “T/S” recommendation; however, only 50% of these patients actually had a preoperative T/S and 33% had a T/C; 6% of these patients were transfused. Thirty-nine percent of patients fell into the “T/C” recommendation category and were further subdivided into 2 U, 4 U, or 6-15 U.
From this algorithm, we created our MSBOS, and it is currently being implemented at the Johns Hopkins Hospital. For example, at our institution, we recommend that a thoracotomy have a T/C for 2 U, a total knee replacement have a T/S, and a cystoscopy have neither T/S nor T/C. Our full schedule can be found in the appendix of the original article in the June 2013 issue of Anesthesiology.2 We have placed emphasis on procedure categories that have low transfusion rates, but high T/C- and T/S-to-transfusion ratios, as these cases do not require preoperative blood orders. For example, these include thyroid/parathyroid, breast, cerebrospinal fluid shunt, cystoscopy, robotic prostate, tonsillectomy, knee and shoulder arthroscopic, and peripheral nerve procedures.
By using this MSBOS to eliminate unnecessary blood orders, we estimated a potential reduction in hospital charges of more than $200,000 and in actual costs of more than $40,000 annually. We also stress the importance of appropriate blood product ordering for improving patient safety, as we discovered that blood was not ordered for some patients who needed it, while it was ordered for some patients who did not. Unnecessary orders can flood the blood bank on the morning of surgery and cause delays for cases that truly do require blood products. Unnecessary crossmatches hold units for a single patient, keeping that product from patients who need transfusion.
It is also important to note that in the event of an unexpected surgical hemorrhage where no blood was ordered, emergency release type-O blood can be used as a backup plan. A mild, delayed, reaction to emergency release blood is expected to occur in approximately 1 in 1,000 transfusions. So if a thyroid surgery, for example, has a risk of transfusion of 2 in 1,000 cases, the risk of a mild transfusion reaction would be only 2 in 1 million cases (2:1,000 x 1:1,000) for a typical patient. Providers should be aware of conditions such as preoperative anemia which might predispose the patient to intraoperative transfusion.
AIMS-acquired data has allowed our group to compile erythrocyte transfusion data on more than 50,000 surgical procedures at our institution to develop an MSBOS with the goal of achieving appropriate preoperative blood product orders. Our MSBOS is currently being implemented, and we anticipate both cost reduction and an improvement in patient safety from this measure.
References
- Friedman BA. An analysis of surgical blood use in United States hospitals
with application to the maximum surgical blood order schedule. Transfusion. 1979;19:268-278. - Frank SM, Rothschild JA, Masear CG, Rivers RJ, Merritt WT, Savage WJ, Ness PM. Optimizing preoperative blood ordering with data acquired from an anesthesia information management system. Anesthesiology. 2013;118:1286-1297.