New guidelines to evaluate and manage chronic kidney disease
Chronic kidney disease (CKD) is a silent disease affecting approximately 10% to 15% of American adults, about the same proportion diagnosed with diabetes. Approximately two of three people diagnosed with CKD will have the major risk conditions—hypertension, diabetes, or both. The CKD prevalence is growing because of the increasing number of people who are diagnosed with type-2 diabetes, which is driven by the aging of the population and the obesity epidemic. CKD imposes an enormous financial and medical burden on the country.
The National Kidney Foundation (NKF) published guidelines for CKD evaluation, classification, and stratification, known as the Kidney Disease Outcomes Quality Initiative (KDOQI), in 2002.1 These guidelines were recently updated as the “KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease” (referred to as the “Guidelines”).2 This article reviews some key aspects of the new Guidelines that have an impact on clinical laboratories.
The clinical laboratory plays a central role in the diagnosis and management of kidney disease. The classic tests related to kidney disease include serum creatinine, creatinine clearance, urea nitrogen, urinalysis, and urinary albumin-creatinine ratio. Another important test is serum cystatin C, an alternative biomarker of kidney function. Given that the leading cause of death among people with CKD is cardiovascular disease, tests for dyslipidemia and diabetes are essential elements of an evaluation. Other tests are important to assess CKD complications, such as anemia, malnutrition, and endocrine, and metabolic changes. An important source of information for laboratories may be found at the National Kidney Disease Education Program (NKDEP) Laboratory Steering Group website.
Why identify CKD?
CKD is a silent disorder. Most patients with CKD feel relatively well and are not aware of the condition. Thus, laboratory testing is crucial to the early diagnosis of CKD. Early intervention and, when appropriate, specialist referral should lead to both economic and medical benefits.
CKD is also caused by a number of heterogeneous disorders. Diseases that affect kidney structure and function such as glomerulonephritis, polycystic kidney disease, and systemic lupus have distinct etiologies, treatments, and prognoses. Most CKD is generally irreversible, and remaining kidney function may be preserved or progression slowed by medication and changes in lifestyle such as tobacco cessation and a healthy diet. CKD may also affect the dosing of drugs that are filtered by the kidneys and then excreted. As many as two-thirds of prescription drugs are cleared by the kidneys.
CKD affects excretory, endocrine, and metabolic functions. Notably, people with CKD may not produce sufficient amounts of the hormone erythropoietin, which stimulates bone marrow production of erythrocytes (red blood cells), and may not be able to convert inactive vitamin D to its active form. Thus, CKD is associated with anemia and secondary hyperparathyroidism and vitamin D insufficiency and deficiency. Other common CKD complications include metabolic acidosis, malnutrition, and bone and mineral disorders. Preparation of those with more advanced CKD stages for dialysis and/or kidney transplantation may improve quality and duration of life as well as lower healthcare costs.
Diagnosis
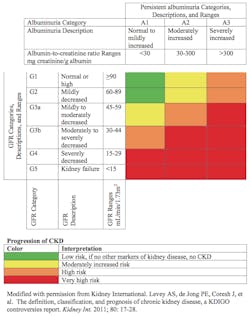
CKD is defined as abnormalities of kidney structure or function that persist for more than three months. There are two criteria, either of which qualifies for a diagnosis of CKD. First, a diagnosis can be made if the Glomerular Filtration Rate (GFR) is less than 60 mL/min/1.73m2. Second, CKD is diagnosed if any of the following are present and persist for more than three months: albuminuria, urine sediment abnormalities, or electrolye and other abnormalities due to tubular damage; abnormalities detected by histology; structural abnormalities detected by imaging.
In the past, the focus was on the estimated GFR (or eGFR). The new Guidelines provide a three-dimensional approach with classification based on cause, GFR, and albuminuria, referred to as CGA (cause, GFR category, and albuminuria category). Including cause as part of the framework allows for separation of diseases with different etiologies, treatments, and rates of kidney disease progression. The new guidelines emphasize the measurement of albuminuria as a risk factor for CKD progression.
The eGFR is a calculated value. The most common calculation currently used in laboratories is based on the MDRD Study equation.3 The newer CKD-EPI equation (applied to adults) is more robust and allows reporting of eGFR of greater than 59 mL/min/1.73m2.4 This allows identification of adults who have mildly decreased eGFR, relative to young adults, of 60-89 mL/min/1.73m2. The new Guidelines recommend use of the CKD-EPI equation in calculating eGFR. Labs are urged to identify the equation used, e.g., MDRD Study equation or CKD-EPI equation, in reporting eGFR, such as in the report name or as a report comment.
In addition, the Guidelines promote applying eGFR using a calculation that incorporates the cystastin C concentration (referred to as eGFRcyst) or creatinine clearance, when relying on the serum creatinine-based eGFR or serum creatinine alone may be less accurate. Performing eGFRcyst is suggested in adults who have eGFR (based on serum creatinine) of 45-59 mL/min/1.73m2 and who do not have other markers of kidney damage. Laboratories that report eGFRcyst are urged to measure cystatin C with a method using calibration traceable to the international reference material. Use of the 2012 CKD-EPI cystatin C equation is supported, and the name of the equation should be specified on laboratory reports.
The lower limit of the reference range, suggesting adequate kidney function, is an eGFR of 60 mL/min/1.73m2, with results that are lower identified as decreased or low. Given that laboratories generally do not collect information about ethnicity, this may lead to a result flagged as low for the eGFR for non-African American patients and within range for African-American patients who have higher eGFR for the same concentration of serum creatinine. Historically, reference ranges for serum creatinine have not been sensitive indicators of diminished kidney function, especially in the elderly. To address this problem, one national laboratory recently revised its serum creatinine reference ranges for adults 50 years and older so that the upper limit corresponds closely to an eGFR of 60 mL/min/1.73m2 and both the eGFR and serum creatinine are flagged similarly.
The identification of CKD is dependent upon clinical laboratory tests, especially serum creatinine and albuminuria. Testing for albuminuria becomes an integral part of every evaluation. Early morning urine specimen collection is preferred. The guidelines list the preference in measurements (in descending order of preference):
- urine albumin-to-creatinine ratio (ACR);
- urine protein-to-creatinine ratio (PCR);
- reagent strip urinalysis for total protein with automated reading;
- reagent strip urinalysis for total protein with manual reading.
When reporting ACR and PCR in untimed specimens, it is recommended that laboratories report the ACR and PCR, respectively, along with the concentration of albumin and protein, respectively. Reagent strip tests should be confirmed by quantitative laboratory measurement. ACR concentrations of ≥30 mg/g on a random untimed specimen should be confirmed with a subsequent early morning urine specimen.
When the eGFR is combined with the albuminuria results, a heat map can be used to classify patients according to prognosis or risk for adverse events (Table 1). Each combination fits into a cell, and each cell has a color designation associated with prognosis, including risk of CKD progression and mortality.
What to expect?
As part of an analysis by a national laboratory, nearly 2.5 million sets of eGFR and ACR results were analyzed to estimate the distribution of these results (Table 2). All patients were assumed to be non-African American, which has the effect of underestimating the eGFR of patients who identify as African-American. This patient cohort is likely to include a disproportionate percentage of individuals who have been diagnosed with CKD, diabetes, or hypertension. Accordingly, the analysis evaluates the distribution of dual results on a large patient population who sought medical care and testing, the population we are most likely to serve in our laboratories. The Guidelines provide a similar analysis of the overall population based on data from the National Health and Nutrition Examination Survey (NHANES).
There was approximately the same percentage of men (49%) as women (51%). The distribution of the dual results was similar for men and women. One striking observation is that among patients with mildly to moderately decreased eGFR (45-59 mL/min/1.73m2), seven of 10 were classified as yellow (moderately increased risk of progression), 23% as orange (high risk of progression), and 8% as red (very high risk of progression). This differentiation, using the dimension of albuminuria, allows for distinctions that were not previously recognized. This has a key impact on patient communication and disease management.
Progression
The Guidelines provide recommendations on the frequency of monitoring patients. CKD progression is defined by a decline in GFR category accompanied by a ≥25% decrease in eGFR from baseline. Rapid progression is defined as a sustained decline in eGFR of >5 mL/min/1.73m2 per year.
Complications
As mentioned, two important complications of CKD are anemia and secondary hyperparathyroidism. Patients with an eGFR of 30-59 mL/min/1.73m2 should be evaluated for anemia at least annually. Patients with lower eGFR should be evaluated for anemia at least semiannually. Patients with an eGFR of 2 should receive testing for calcium, phosphate, intact parathyroid hormone, and alkaline phosphatase. When the intact parathyroid hormone concentration exceeds the upper limit of the reference range, patients should be evaluated for calcium, phosphate, and vitamin D deficiency.
The Guidelines identify an issue with BNP/NT-proBNP and troponin assays. These test results must be interpreted with caution among patients with an eGFR 2. Congestive heart failure and fluid overload are more prevalent among patients with lower levels of kidney function. Unfortunately, these tests become less reliable as predictors of fluid overload and heart failure in these patients. Different laboratory and clinical criteria must be considered when the eGFR is 2.
The Guidelines comprise 150 pages, and it is impossible to capture all of the recommendations that impact the laboratory in this article. Our purpose is to summarize this important document and to enlist your participation in providing the laboratory testing that is essential to evaluate and manage patients with kidney diseases of all types. By working together, we can provide physicians and their patients with CKD with information that will lower healthcare costs and improve healthcare outcomes.
References
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266.
- National Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Intn Suppl. 2013;3:1-150.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461-470.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612.
About the Author

Harvey W. Kaufman, MD
a board-certified pathologist, is Senior Medical Director at Quest Diagnostics. A 29-year veteran of Quest Diagnostics, Kaufman was an original and still active member of the Laboratory Working Group of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH (formerly the National Kidney Disease Education Program).