Glycemic biomarkers as tools for diagnosis and monitoring of diabetes
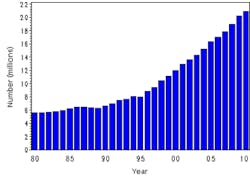
Recent statistics on the diabetes epidemic are staggering. The incidence of diabetes is rising, and based on data released in the American Diabetes Association (ADA) 2011 National Diabetes Fact Sheet, 8.3% of the population of the United States now has diabetes (www.diabetes.org). The prevalence of type 2 diabetes is on the rise and has more than doubled since 1993.1 In the United States today, according to data compiled by Centers for Disease Control and Prevention (CDC) there are at least 18.8 million diagnosed cases of diabetes and seven million undiagnosed cases (Figure 1). About 1.9 million new cases are diagnosed each year, and there are considerable racial and ethnic differences, with rates being highest for non-Hispanic blacks (12.6%) as compared to whites (7.4%), Asian Americans (8.4%) and Hispanics (11.8%). Today, diabetes-associated complications remain the leading cause of heart disease- or stroke-related deaths and are associated with long-term damage including the failure of organs such as eyes and kidneys.
Figure 1. Number (in millions) of civilian, noninstitutionalized persons with diagnosed diabetes, United States, 1980–2010 Source: U.S. Department of Health and Human Services. Center for Disease Control and Prevention. http://www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm
Although simply defined on the basis of hyperglycemia, diabetes mellitus today is known to be a highly heterogeneous disease. Based on etiology/pathogenesis, diabetes can be classified in two major categories. Type 1 diabetes is characterized by absolute insulin deficiency due to autoimmunity leading to β-cell destruction and can be identified by serological markers of autoimmunity (islet cell autoantibodies). It accounts for 5% to 10% of all diabetes cases. Type 2 diabetes is caused by insulin resistance and lack of compensatory insulin secretory response. In contrast to type 1 diabetes, type 2 diabetes is highly prevalent and accounts for 90% to 95% of all diabetes cases. The high impact of this chronic disease in the U.S. healthcare system requires more reliable lab tests for early accurate diagnosis and for long-term monitoring.
In the past, the diagnosis of diabetes was based on either fasting plasma glucose or two-hour plasma glucose level in the oral glucose [75 g] tolerance test (OGTT). Recently, the International Expert Committee (2009) and the ADA Clinical Practice Guidelines (just published in 2013) recommended the use of glycated hemoglobin (HbA1c) to diagnose diabetes.2-3 This article will review the current state of the laboratory testing with special focus on the role of HbA1c and other emerging glycated protein biomarkers, including fructosamine and glycated albumin (GA), in the diagnosis and long-term monitoring of diabetes.
Screening and diagnosis of diabetes mellitus
Glucose measurement and glucose tolerance tests. For decades the diagnosis of diabetes mellitus (type 1 and type 2) was made on the basis of an elevated fasting glucose level. The ADA proposes that fasting plasma glucose (FPG) should be measured in all asymptomatic people ≥45 years of age and screening should be considered at a younger age in individuals at increased risk for diabetes. The three-to-five hour oral glucose tolerance test, once the gold standard for diagnosing diabetes, is currently not recommended either by the ADA or the International Expert Committee.2-3 However, both the ADA and International Expert Committee continue to recommend use of the two-hour oral glucose challenge test, especially in gestational diabetes mellitus (GDM).
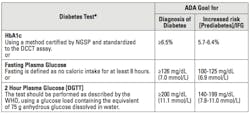
A fasting glucose concentration of ≥126 mg/dL on more than one occasion is considered diagnostic of diabetes. However, in the absence of clinical symptoms a glucose level between 100 mg/dL and 125 mg/dL indicates an increased risk for diabetes (prediabetes) and is referred to as impaired fasting glucose (IFG). Based on current recommendations from the National Diabetes Data Group, the OGTT must be done after three days on a diet containing a minimum of 150 g of carbohydrates per day. The following day, after an overnight fast, 75 g of glucose is given in water. (Five samples are generally collected, one before the glucose loading and again at every 30 minutes up to two hours after loading.) According to the ADA and the International Expert Committee diagnostic criteria for abnormal glucose tolerance, a blood glucose level of ≥200 mg/dL at two hours is diagnostic of diabetes and a level of ≥140 mg/dL indicates impaired glucose tolerance (IGT) (Table 1).2,3 The latter is a heterogenous category that includes many people who may have a subsequent normal oral glucose tolerance test, and almost 30% of patients who develop diabetes may not exhibit impaired glucose tolerance.4
Table 1. Criteria for the diagnosis of diabetes and of increased risk for diabetes [prediabetes]/Impaired Fasting Glucose [IFG] adopted from ADA—Clinical Practice Recommendations [Diabetes Care, 36 [S1], 2013]
Thus, the OGTT results often may be nondiagnostic. Furthermore, in spite of standardization, many factors, including noncompliance for fasting and inability to tolerate the glucose load, among many other factors, influence the final test outcome. During the OGTT, insulin levels normally peak at one hour, reaching 50 mU/mL to 130 mU/mL and returning to below fasting concentrations in two hours. The clinical utility of insulin measurement is limited, primarily because when fasting glucose is elevated, β-cell responsiveness decreases, and when the fasting glucose level is normal, late hyperinsulinism may occur in type 2 diabetes or in the early phase of type 1 diabetes.
Glycemic biomarkers. Glycemic biomarkers are important tools to monitor glycemic control.5 Measurement of glycated proteins, primarily HbA1c, has been widely used for routine long-term monitoring of glucose control and as a measure of risk for the development of diabetes complications. The test accurately assesses the mean blood glucose level during the preceding two to three months; therefore, it complements the more traditional measures of glucose control (blood or urine glucose testing). In addition to hemoglobin, other proteins in blood can also be glycated, and glycated proteins such as fructosamine and glycoalbumin (GA) can also be measured and used as an estimation of glucose control.5 Today the use of HbA1c is also recommended by the ADA and other organizations for diabetes screening and diagnosis. However, it has some limitations, indicating the need for the use of other glycated protein biomarkers.
Hemoglobin A1c. Hemoglobin A1c was first discovered in 1969 as an abnormal hemoglobin fraction in blood from diabetes patients. It was later shown that glucose binds to hemoglobin in red blood cells, and the term “glycohemoglobin” is applied to a number of chemically distinct hemoglobin components that are generated when glucose binds to hemoglobin.
The glycation of hemoglobin occurs at several amino acid residues and, as a result, several adducts of hemoglobin A (HbA) and various sugars are formed by the nonenzymatic post-translational glycation process. This process involves the formation of a labile Schiff base intermediate followed by the Amadori rearrangement. The reaction is slow, continuous, and irreversible, and the reaction rate depends on the ambient glucose concentration. Minor components of HbA were first recognized because of the differences in their electrical charges and were called “fast hemoglobin” as they migrated at a faster rate than an entire HbA molecule when placed in an electrical field. These minor components were labeled in order of elution from an ion exchange column as HbA1a,, HbA1b, and HbA1c. The most important HbA component in diabetes is HbA1c, which has glucose attached at the N-terminal-amino groups of both β chains of hemoglobin.
Various methods for the measurement of HbA1c have been described and reviewed.6 These methods can be classified in two major categories. One category is based on separation and detection of HbA1c on the basis of charge differences and includes ion-exchange chromatography, high performance liquid chromatography (HPLC), and agar gel electrophoresis. The other category includes methods that separate components based on the structural differences and include boronate affinity chromatography.5 Immunoassays for HbA1c also have been described.7-8 Among the methods, charge-based immunoassays specifically detect Hb-A with glucose attached to the amino terminal valine of beta chains, while ion-exchange chromatography quantifies total HbA1c and other Hb-glucose adducts such as glucose lysine and glucose α-chain N-terminal valine adducts.
Because of their ability to process a large number of samples and their superior precision, automated HPLC and automated immunoassays are among the most widely used methods. Results of the various methods demonstrate excellent correlation. There is no convincing data to show that one method is superior to the other, as practically all commercial methods are standardized to a common reference standard. Laboratories should use only the HbA1c methods that are certified by the National Glycohemoglobin Standardization Program (NGSP) as traceable to the Diabetes Control and Complications Trial (DCCT).9 The rationale to standardize the HbA1c methods to DCCT equivalent values was that DCCT had determined the relationship between HbA1c values and long-term outcome risks in patients with diabetes.
Besides the standardization of various methods, one may also be aware of the clinical situations in which HbA1c may not provide an accurate estimation of glycemic control. Glycation of HbA1c not only depends on average glycemia but also on the rate of production or destruction of RBCs. Hence, its levels can be impacted by the conditions that affect RBC life span. Conditions linked to short RBC life span, e.g., in patients with hemolytic anemia and splenomegaly,10 will have false low HbA1c levels independent of the mean serum glucose. Also, HbA1c levels can be false high in situations that increase the production of RBCs, as in patients with chronic kidney disease who receive erythropoietin for anemia or in patients who receive a blood transfusion.11 In brief, HbA1c levels are shown to be positively associated with hemoglobin levels and are negatively associated with erythropoietin dose. Furthermore, presence of hemoglobinopathies such as sickle cell or other hemoglobin variants (C & E) as well as iron status can affect the HbA1c levels.12,13 These limitations in HbA1c indicate the need of other glycated proteins such as fructosamine and glycated albumin that are not affected by hemoglobin level alterations.
Glycated proteins. Like glycohemoglobin, glucose molecules are joined to protein molecules through a nonenzymatic glycation mechanism to form stable ketoamines termed fructosamine. All serum proteins can be glycated, so all glycated proteins are fructosamines. Albumin is the most abundant serum protein, and it contains multiple lysine residues, all of which can be glycated. Therefore, GA concentrations account for ~90% of fructosamine measurements. Just like glycohemoglobin, fructosamine and GA measurements serve as an index of the mean concentration of glucose in the blood during the preceding several weeks. However, because of rapid turnover of serum proteins compared to hemoglobin, the fructosamine and GA levels reflect glucose control over a shorter period of two to three weeks rather than six to 10 weeks for glycohemoglobin.
Several assays designed to measure glycated serum proteins (fructosamine and GA) have been described. These include colorimetric assay for fructosamine, affinity chromatography (including HPLC), and immunoassays using antibodies for measuring glycated albumin.14-15 Among these, the colorometric assay (introduced in 1983), based on the ability of ketoamines to reduce nitroblue tetrazolium (NBT assay) to produce a compound that is detected by absorption at 525 nm, has been adapted for many automated chemistry analyzers and is the most commonly used method.16 As these assays detect glycoproteins, total protein concentration is likely to influence fructosamine and GA results. Abnormal protein turnover influences glycated protein values. There are clinical situations in which fructosamine or GA should not be used, such as for thyroid disease (as in thyrotoxic or hypothyroid patients in whom protein turnover is increased or decreased). Levels are also influenced by low albumin levels, as seen in patients with protein-losing enteropathy, nephritic syndrome, or liver failure. Total protein or albumin concentrations seem to correlate with fructosamine levels. The current recommendation, therefore, is to normalize fructosamine results to a given serum albumin or total protein concentration. Also, with a colorimetric analysis, bilirubin, hemolysis, and lipemia will likely interfere in the measurement.
Despite these limitations, measuring fructosamine or GA provides some advantages over measuring glycohemoglobin A1c as they are not affected by RBC life span, and the glycated protein levels respond more quickly to changes in glycemic control than glycohemoglobin A1c. However, these assays are currently used only to complement glycohemoglobin A1c assays to manage diabetic patients when the detection of short-term metabolic changes is required.
Long-term monitoring of diabetes mellitus
The effective clinical management of diabetes requires accurate measurement and monitoring of blood glucose levels. Traditionally, plasma glucose has been the lab test used for monitoring the patient with diabetes. Findings from the Diabetes Control and Complication Trial demonstrate that maintaining blood glucose close to normal reduces diabetes complications.17 This study compared the standard versus intensive blood glucose control on the complications of diabetes. Keeping HbA1c close to normal (6% or less) was used as a measure of intensive control. Today, use of HbA1c is accepted as a long-term monitoring tool for the management of diabetic patients and is used in conjunction with plasma or finger-stick glucose testing. Portable glucose meters are routinely used either in physician offices or by patients at home. Day-to-day management as guided by self-monitoring of blood glucose by patients with the use of glucose meters is a common practice today.
Several studies have explored the relationship between HbA1c and chronic glycemia and have supported the association of HbA1c with average glucose levels over the preceding five to 12 weeks.17 These studies have relied on infrequent measures of capillary glucose levels, calling into question the validity of their assessment of chronic glycemia. More recently, an international multicenter study examined the relationship between average glucose, as assessed with a combination of continuous glucose monitoring and frequent finger-stick capillary glucose testing and HbA1c levels over time, and demonstrated a close relationship between the two. Based on this relationship HbA1c levels can be used to calculate an estimated average glucose (eAG) (AGmg/dL = 28.7xA1c-46.7, R2 = 0.84, P<0.0001).18 The linear regression equations did not differ significantly across subgroups based on age, sex, diabetes type, race, or smoking status. Now HbA1c levels can be expressed and reported as eAG for most patients with type 1 or type 2 diabetes. The calculated eAG level gives healthcare providers a more useful index of chronic glycemia. The International Federation of Clinical Chemistry and Laboratory Medicine and the International Diabetes Federation consensus guidelines have endorsed reporting HbA1c values along with the calculated eAG level.19
To conclude, it is fair to say that the diagnosis of diabetes is changing. Currently HbA1c is widely used as a glycemic marker for long-term monitoring of diabetes, as it provides valuable information about the degree of glucose control during the previous eight to 12 weeks. It is also being used as a primary treatment target, as it has been shown to have close association with diabetes complications. Furthermore, its value can be translated into eAG values, providing valuable information to clinicians in monitoring patients with diabetes.
Recently, HbA1c has been incorporated into diabetes diagnosis, and is recommended by the International Expert Committee based on advances in instrumentation and standardization that make it an accurate and precise marker. This represents a significant change in diagnostic lab testing for diabetes. However, it should be reiterated that there are some limitations, and HbA1c may not provide accurate information in certain clinical situations that affect RBC life span—for example, certain hemoglobulinopathies (hemoglobin S, C, or E), anemia, and renal dysfunction. If a discrepancy between blood glucose and HbA1c is observed, one must consider the measurement of extracellular glycated proteins (fructosamine or GA), as these are not affected by RBC life span or iron status. However, their relationship to average glucose and their prognostic value to diabetes complications are not clear, as in the case of HbA1c.
References:
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. 2011 National diabetes fact sheet.
- International expert committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes care. 2009;32:1327-1334.
- Standards of medical care in diabetes—2013. Diabetes care. 36:S11-66.
- Balkau B, Eschwege E. Repeatability of the oral glucose tolerance test for the diagnosis of impaired glucose tolerance and diabetes mellitus. Diabetologia. 1991;34.
- Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM. Tests of glycemia in diabetes. Diabetes Care. 1995;18:896-909.
- John WG, Bullock D, MacKenzie F. Methods for the analysis of glycated hemoglobins: what is being measured. Diabetic Med. 1992;9:15-19.
- Javid J, Pettis P, Koenig RJ, Cerami A. Immunologic characterization and quantification of hemoglobin A1C. Br J Haematol. 1978;38.
- Fleming JK. Evaluation of HbA1c on the Roche COBAS Integra closed tube system. Clinical Biochemistry. 2007;40:822-827.
- Goldstein DE, Little RR. Bringing order to chaos: the National Glycohemoglobin Standardization Program. Contemp Int Med. 1997;9:27-32.
- Panzer S, Kronik G, Leckner K, et al. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood. 1982;59:1348-1350.
- Spencer DH, Grossman B, Scott MG. Red cell transfusion decreases hemoglobin A1c in patients with diabetes. Clin Chem. 2011;57:344-346.
- Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clinical Chemistry. 2001;47:153-163.
- Sofronescu A, Williams LM, Andrews DM, Zhu Y. Unexpected hemoglobin A1c results. Clinical Chemistry. 2011;57:153-156.
- Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153-2163.
- Shima K, Ito N, Abe F, et al. High performance liquid chromatographic assay of serum glycated albumin. Diabetologia. 1988;31:627-631.
- Johnson RN, Metcalf PA, Baker JR. Fructosamine: a new approach to the estimation of serum glycosylprotein: an index of diabetic control. Clin Chem Acta. 1983;127:87-95.
- Rohlfing CL, Weidmeyer HM, Little RR, et al. Defining the relationship between plasma glucose and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275-278.
- Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473-1478.
- Consensus Committee: Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007;30:2399-2394.