In this paper, I shall describe what dry hematology is, why and how it has been developed, what it does and how it works, and the point of care market segments it best serves, as well as the part my associate Stephen C. Wardlaw, MD, and I have played in its invention and development. I will compare and contrast the attributes of presently available point of care dry hematology analyzers with the presently available point of care wet hematology analyzers.
What is dry hematology?
“Dry hematology” or other “dry testing” may be defined as analyses carried out without the addition of any significant diluent or liquid reagents. The most familiar example of “dry testing” is probably the urine dipstick. The spun hematocrit, the HemoCue®, and the QBC Centrifugal® systems are currently FDA cleared. Because of its freedom from the need for complex fluidics and heavy liquid reagents/diluents, and its resultant relative compact size and ease of use, dry hematology has found an important role in point of care testing. Ongoing research and development in the field of dry hematology strongly point to its bright future and an increasing role in point of care diagnostics.
Why is dry hematology needed at the point of care?
Arguably the most important and frequently ordered diagnostic blood test at the point of care is the complete blood count or CBC. A recent case involving a septic twelve year-old boy was opined about in the New York Times. The boy’s death was due in part to the late delivery, after he had been discharged from the emergency room, of his abnormal CBC results. This delay contributed to the failure of the healthcare provider and system to associate the boy’s abnormal results with the history and findings of the patient.1
A patient’s healthcare needs are best met when accurate hematology results, regardless of the method employed, are immediately available to the physician, while he or she is still in the presence of the patient, thereby allowing the physician to make informed decisions on triage, additional testing, and treatment. Benefits accrue not only to patients, who will have improved outcomes, but to the physicians, who can more confidently diagnose, triage, and treat the patients. Society also benefits through decreased costs and improved quality of medical care. Potentially dangerous, expensive, and often unnecessary medications may be avoided, and life-saving medication or treatment for illnesses such as sepsis, severe anemia, and thrombocytopenia can be instituted immediately. Because of their relative simplicity of operation, compactness, and subsequent utility at the point of care, dry hematology methods are logical candidates to meet this need.
Centrifuge-based dry hematology: determination of basic hematology parameters
Dry hematology performed at the point of care was invented in 1929 by Dr. Maxwell Wintrobe, considered by many the “father of modern hematology,” with his invention of the spun hematocrit.2 It was his invention and teachings, through multiple editions of his eponymous text Wintrobe’s Clinical Hematology, promoting the visual examination of the buffy coat of centrifuged blood, that led Wardlaw and me to invent, thirty-six years ago, the first dry hematology system that delivered multiple hematologic parameters.3 Dry hematology was not our goal. It was our solution, as physician/laboratorians, to satisfy our need to have a means of obtaining rapid, robust, reliable, point of care hematology results. At the time, I was a practicing internist in a rural community as well as a director of a radio immunoassay laboratory at a university. Wardlaw was a clinical and anatomic pathologist directing the hematology laboratory. He was interested in and highly proficient in medical instrumentation.
Our centrifugally based hematology technology, which we called the Quantitative Buffy Coat Analysis, later shortened to QBC, works by expanding the buffy coat, a thin buff-colored layer of cells and platelets that come to rest on top of the packed red blood cells when blood is centrifuged, as in a spun hematocrit. The expansion allows linear measurement of the layers and yields a hematocrit, hemoglobin, Mean Corpuscular Hemoglobin Concentration (MCHC), total white blood cell count as well as a two-part differential (absolute granulocytes and lymphocytes plus monocytes) and a platelet count derived from the total platelet volume. These parameters were those that primary care physicians treating outpatients needed the most. I was now able to immediately detect or distinguish anemia from other causes of pallor, thrombocytopenia-induced bruising from spousal abuse trauma, granulocytopenia, severe bacterial infections, and other significant illnesses. This new availability of real time hematology results was transformative to my practice and significantly increased my satisfaction practicing medicine, especially in a location remote from a laboratory.
Following our proof of concept, with a mixture of pride and anxiety, I called Dr. Wintrobe and described this new technology that he had inspired. He responded with a few moments of silence and then said, “I want to meet you and Dr. Wardlaw.” He invited us to his home. We became friends with Dr. Wintrobe and worked closely with him developing an expert system to aid physicians in interpreting the hematological results4 and creating a pictorial view of buffy coat results.5
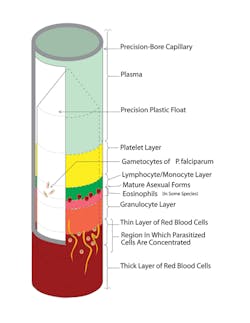
The QBC centrifugal-based hematology method utilizes about 50 to 80 microliters of capillary or venous blood; an exact volume is not required, since the analyzer measures and calculates the volume during analysis. It is enabled by the linear expansion of the buffy coat by the presence of a close fitting cylindrical insert or float inside a dried acridine orange-coated capillary tube. The float by virtue of its density settles on top of the centrifuged packed red cells and is surrounded by three discernable and now measurable layers of the buffy coat (Figure 1 and Figure 2). The tenfold expansion is the result of the insert occupying exactly 90% of the cross-sectional area of the tube. The layers’ lengths, easily visualized by their distinct fluorescence due to their uptake of acridine orange, are measured, and their lengths are converted to the reported parameters by coefficients specific for each cell type and platelets, corrected for the volume of the blood.
Figure 2. This cutaway view shows the hematoparasites. Malaria, babesia, filarial and leishmania as well as borellia may be visualized.
The hemoglobin concentration is calculated by determining the density of the centrifugally packed red cells by measuring the depth of the float in the packed red cells and correcting its depth and by accounting for the floatation forces of the buffy coat. The packed red cells’ density is a function of their MCHC, and knowing the hematocrit enabled the determination of the hemoglobin concentration. The simplicity of the method was that it required no additional reagents or measurements, just additional calculations. Just as spectrophotometric hemoglobin determinations are based on optical density, the QBC method is based on the mass density of the hemoglobin. A more detailed description of this FDA-cleared methodology and its history is available.6–9
This QBC, initially manually measured, is now performed by an automatic reader built into the centrifuge. It has become widely used throughout the world in physicians’ offices and in military field locations as well as most surface ships and submarines in the United States Navy. The addition of species-specific packing coefficients specific for different mammalian species led to the widespread utilization of the method in veterinary medicine.10
Our present research on centrifugal hematologic methods is divided between improving the functionality of the centrifugal hematology system by utilizing modern imaging and telecommunication. Very recently the added ability to capture and transmit full images of the centrifuged tube enabled the optional remote analysis of the images and remote quality control, thereby potentially easing physicians’ burden in meeting regulatory requirements.11
QBC detection of malaria and other hematoparasites
Soon after we invented the quantitative buffy coat system, I observed my veterinarian perform a labor-intensive filtration test to check my pet dog for canine heart worm. Wardlaw and I immediately realized the QBC’s potential application for the detection of circulating hematoparasites, which we hypothesized might, because of their density or the density of the infected red cells, be localized by centrifugation to the annular free space surrounding the float. The first dog checked had frightening (under the microscope) undulating microfilaria concentrated in the buffy coat region. This test was used for years but has been superseded in dogs by antigen tests. It is still widely used for human filariasis in Africa.12
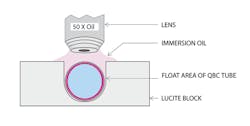
We waited several years for a potential malaria-infected patient. Having no success (as it were), we settled for a mouse allegedly infected with malaria. We observed that the mouse had malaria as well as unexpected trypanosomiasis. Encouraged by the success of the technology, we began field studies with Drs. Andrew Spielman and Curtis Patton, and shortly after the QBC received FDA clearance for diagnosing malaria. The QBC malaria test uses the same tube but requires visual examination utilizing a fluorescence microscope.13 The hematoparasites and red blood cells infected by them come to rest in the buffy coat area, or slightly below it, due to their density being lower than the non-infected red cells. Since they are displaced by the float to the easily inspected twenty-nine micron free space surrounding the float, they can be visualized, by placing the centrifuged tube in a notched holder and examining the tube using a 50X oil objective on an epi-illuminating microscope, due to the parasites’ uptake of acridine orange and the resultant fluorescence14 (Figure 2 and 3). This technology has also proved useful for diagnosing filariasis, babesiosis, and trypanosomiasis (sleeping sickness and Chagas disease both congenital and in compromised patients), and African relapsing fever due to borrelisosis.15 A microscope attachment, which converts a standard microscope to an epi-illuminating UV scope, was developed by Wardlaw.16
Shortly after the QBC Malaria test was approved I saw a patient in my Connecticut office who was visiting from Tanzania. He was complaining of fatigue, headaches, and intermittent fever of several months duration. He stated that he had been tested repeatedly for malaria and was negative. I examined him and found no significant physical findings but did elect to look at his blood with a QBC. I saw actively moving fluorescent intraerythocytic organisms moving purposefully, not by Brownian motion. This was the first and to this day only time I personally used the QBC to look for hematoparasites in a patient. I had no idea what the parasite was. I asked Wardlaw and another physician, who confirmed my conclusion that there were intraerythrocytyic organisms moving in some of the red blood cells, but they too could not identify them. I called Dr. Patton and asked him if he could drive to my office and offer his opinion. He came, looked at the specimen under the microscope, smiled, and asked, “What do you think vivax means? Vivax, in Latin, means lively!” None of my colleagues nor I had ever seen a live Plasmodium vivax.
The diagnosis of hematoparasitic disease at the point of care by direct observation of the pathogen is an especially important benefit of dry hematology, especially when routine hematology results may be obtained at the same time using a QBC reader with the same disposable for no additional expense. The presence of anemia and thrombocytopenia can be important clinical findings affecting treatment of patients diagnosed with malaria.
Detection and counting circulating cancer cells
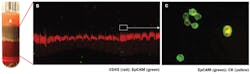
Our success with the use of the QBC technology to detect hematoparasites led one of our post doctoral fellows, Dr. Paul Fiedler, to suggest that we try it to see if we could detect circulating cancer cells. He took a swab of his buccal mucosa and briefly swirled it in a seven ml tube of his blood. The epithelial cells were readily visible right where the hematoparasites were. We decided to utilize a fluorescently tagged antibody against epithelial surface antigens and soon determined that we could detect and quantify circulating carcinoma cells in patients’ blood. This application of QBC requires using a sample a hundred times larger than the approximately seventy microliter contained in the QBC tube. A ten ml float containing tube and instrument to detect image, count, and phenotypically characterize circulating tumor cells is now available as a research technology and may prove useful at the point of care in oncologists’ offices to determine tumor response to chemotherapy (Figure 4).17,18
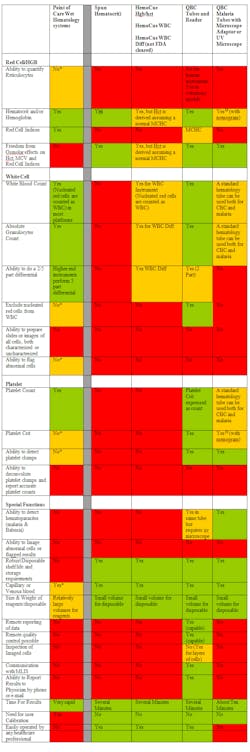
The HemoCue® uses a modified azomethemoglobin hemoglobin method, where a 10 µl blood sample is introduced into an extremely thin cuvette containing all necessary reagents. There is also a HemoCue method for total white blood count (WBC), which is FDA cleared, that images stained cells within a similar chamber, and one platform that reports a five-part differential that is not FDA cleared. Both lack the ability to distinguish WBCs from nucleated red bloods. The HemoCue methods do report accurate hemoglobin as it is determined spectrophotometrically, but the hematocit result is a calculation based upon an assumed MCHC and therefore has no additional informational value. Obtaining hemoglobin and WBC results employing the HemoCue methods requires two different cuvettes and readers; neither reports a platelet determination. The method is easy to use, and the instrument is compact and requires no user calibration. The requirement of two instruments and disposables to obtain hemoglobin and WBC and the absence of platelet results limit its utility as described in Table 1 and Table 2.
Green= Fully performs the atribute
Yellow = Adequate with Reservations
Red = Not good or not available
* Most point of care hematology instruments do not have this feature
Table 1. Attributes of dry hematology methods compared and contrasted to point of care wet hematology systems
Green= Ideal
Yellow = Adequate with Reservations
Red = Not needed or not applicable
Table 2. Point of care markets and the dry hematology methods of choice
Conclusion
The universe of point of care sites is large, and their hematological needs vary according to their size, location, and availability of medical technologists, reagents/disposables supply, and repair logistics. At the present time there are still needed features that
are not yet present in available methods both wet and dry. The utilization of and further development of appropriate point of care hematology diagnostic methods can significantly improve patient care. That is the “point” of point of care diagnostics.
References
- Dwyer J. Death of a boy prompts new medical efforts nationwide. New York Times. 2012;10.25:A6.
- Wintrobe MM. A simple and accurate hematocrit. J Lab Clin Med. 1929;15:287-289.
- Wardlaw SC, Levine RA. Quantitative buffy coat analysis (QBCA): a new laboratory tool functioning as a screening complete blood cell count. JAMA. 1983;249:617-620.
- Adrion RF, Curry JW, Levine RA. Hematology—diagnosis apparatus employing expert system technology. US patent. 5,023,785. June 11, 1991.
- Wintrobe M, Levine RA, Wardlaw S. Buffy coat analysis, a pictorial review (privately published by Becton Dickinson), 1983.
- Levine RA, Wardlaw SC. A new technique for examining blood. American Scientist. 1988;76(6):592-598.
- Wardlaw SC, Levine RA. Material layer volume determination. US patent. 4,027,660. June 7, 1977.
- Levine RA, Wardlaw SC. Method for measuring hemoglobin. US patent. 4,843,869. July 4, 1989.
- Wardlaw SC, et al. Method for increasing agglutination of groups of cells to produce improved cell layer interface in centrifuged blood sample using antibodies. US patent. 4695,553. September 22, 1987.
- Levine RA,Wardlaw SC, Hart AH. Quantitative buffy coat analysis of blood collected from dogs, cats and horses. JAVMA. 1986:189(6);670-673.
- Levine JD, Levine RA, Wardlaw SC, Stout C, Clipp DA. Method and apparatus for remotely performing hematologic analysis utilizing a transmitted image of a centrifuged analysis tube. US patent application. 20110200239. August 18, 2011.
- Salmani MP, Peerapur PBMBV. Comparative Study of Peripheral Blood Smear, QBC and Antigen Detection in Malaria Diagnosis, J Clin Diag Res. 2011;5(5):967-969.
- Levine RA, Wardlaw SC, Patton CL. Detection of hematoparasites using quantitative buffy coat analysis tubes. Parasitology Today. 1989:5(4);132-134.
- Spielman A, Perrone JB, Teklehaimanot A, Balcha F, Wardlaw SC, Levine RA. Malaria diagnosis by direct observation of centrifuged samples of blood. Am J Trop Med Hyg. 1988;39(4):337-342.
- Cobey FC, Goldbarg SH, Levine RA, Patton CL. Short report: detection of borrelia (relapsing fever) in rural Ethiopia by means of the quantitative buffy coat technique. Am J Trop Med Hyg. 2001;65(2):164-165.
- Rathbone R, Wardlaw SC. Adaptor for microscope. US patent. 5,198,927. March 30, 1993.
- Rimm DL, et al. Method for assaying whole blood for the presence or absence of circulating cancer or other target cell fragments. US patent. 6,670.197. December 30, 2003.
- Ramirez AB, et al. The RareCyte™system for enumeration of circulating tumor cells that retains all nucleated cells for analyses and does not rely on capture of proteins expressed on cells. http://rarecyte.com/pdf/AACC_2011_poster_large.pdf. Accessed December 21, 2012.
- Manion KL, et al. Apparatus for measuring blood constituent counts. US Patent. 5,132,087. July 21, 1992.