Improving efficiency in the pathway from collection to care
For many years, it was simply accepted that the microbiology lab could not be automated in the same way that the clinical chemistry and hematology labs had long been automated. Microbiology is a complicated discipline, as labs face a wide diversity of pathogens that continue to evolve and modify their resistance capabilities. The diversity of the specimen types ads to the complexity with many challenges in sample preparation. Each organism requires specific growth conditions, media, and incubation times. Therefore, microbiologists have been faced with the challenge of accommodating more testing capabilities, increasing the accuracy of identifying pathogens, and enchancing the efficiencies in the lab.
Automation has started to change the microbiology lab, however, and the best is yet to come. But automation alone is not the solution. The complex problems the microbiology lab faces, as well as healthcare in general, require a three-pronged solution focusing on people, process, and technology.
There are many dynamic forces within healthcare pushing the microbiology lab to reduce waste and inefficiency in the pathway from sample collection to results. Clinicians need faster turnaround times (TATs) for lab results. Lab and hospital administrators must meet new stringent standards and performance metrics for electronic health records (EHRs), efficiency, and patient care. And of course, the labor shortage has made automation a must. It typically takes five years to properly train a good bench microbiology scientist. Waves and waves of these professionals are approaching retirement age, with very few new scientists entering the field to replace them. As they retire, the profession will lose thousands of years of experience and institutional memory. Meanwhile, the microbiology lab faces the challenges of improving efficiency, clinical outcome, and operational cost.
A holistic approach: people, process, and technology
The pathway from sample collection to lab results in many hospital microbiology labs includes sample “batching,” which is a common practice in U.S. labs where specimens sit idle for several hours or even overnight before a large enough “batch” is collected to begin inoculating agar plates for incubation. If a very sophisticated piece of automation, such as an automated plate streaker, is further downstream from this artificial bottleneck, all the potential efficiency provided by automation could be lost because of an institutional inefficiency further upstream in the pathway from the automated task. Thus, automation in and of itself is not the solution.
Automation is a piece of the solution. But the two other key pieces are people and process. This holistic approach falls under the umbrella of performance solutions, which include improving people, process, and technology. The latest piece of automated equipment cannot provide overall lab performance improvement without a change in people and in process as well.
It should be stressed that the “people” aspect of the equation does not mean staff reductions. In dozens of process improvement assessments conducted at microbiology labs, staff reductions have never been an outcome. By improving people, we mean maximizing staff experience- optimizing their knowhow, removing them from tedious tasks, and reducing repetition. During a recent microbiology lab assessment, analysis revealed that 69 people were involved in specimen processing. Not only is this a waste of precious human resources, but it introduces too much variability into what should be a uniform system, as well as increasing the likelihood of human error.
In the March 2012 issue of MLO, Dr. Joseph Campos of the Children’s National Medical Center described how this holistic approach involving people, process, and technology dramatically improved the turnaround times in his lab.1
“In just two years after implementation of the Lean program, the CNMC microbiology lab has achieved significant improvements in efficiency. The early results are a streamlined staffing and workflow system and a lab designed to maximize both personnel and equipment. Because the Lean system is based on the philosophy of constant improvement, I’m confident that we will continue to see and adopt new ideas and processes that will guide our lab for years to come,” wrote Dr. Campos.
Automation was key to his lab’s success, but it was just one piece of the puzzle. Dr. Campos redesigned his lab to reduce inefficiency and added shifts—without hiring new staff—to avert bottlenecks in sample processing and plate readings that occurred every morning. And the ongoing implementation of LEAN Six Sigma provides the CNMC lab with continuous process improvement.
Bad habits don’t afflict only people. They also impact groups of people, labs, hospitals, and entire hospital systems. Does the typical microbiology labmonitor TATs? Based on lab workflow assessments at hundreds of hospitals, the vast majority would answer “No.” Rather than benchmarking their TATs, most labs equate TATs with their protocol and the time it takes to complete that protocol. Of course, this protocol could be efficient or incredibly inefficient, so they never actually have an understanding of how they can improve. Likewise, most labs don’t track plate streaking errors. If there is no growth after 18 hours, they simply start again. Was there no growth because the patient isn’t infected, or because the plate was poorly streaked? If it is the latter, that patient could be getting the wrong therapy or no therapy, and no one would know for sure until at least 18 hours later!
This is how inefficiencies in the microbiology pathway become institutionalized and poor performance goes unrecognized. It must be emphasized that this is human nature, not “laboratorian nature.” All humans do this, in our personal lives and professional lives. Often, it takes an outside observer to help us see how we can improve.
The holistic approach to improvement can be measured in more than reduced TATs. In scores of lab performance assessments, lab staff report reduced stress and increased productivity, fewer errors, capacity optimization, improved sample flow through, and a reduction in work-in–process, which all microbiologists know is a major cause of delayed results.
The microbiology lab pathway: introducing automation
Microbiology automation has come a long way. Not that long ago, some labs purchased first generation plate streakers and gram strainers but stopped using them because techs could do the job faster. That may once have been true, but the second generation of these products has made huge leaps in speed, reproducibility, and accuracy.
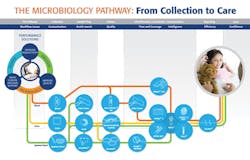
Figure 1 represents the holistic approach to the microbiology pathway, an approach that increases productivity, reduces stress and errors, and improves patient care.
Figure 1.
While this pathway is not yet “hands free,” labs equipped with automated devices have witnessed a dramatic decrease in points of human intervention. As we know, every such point brings with it the risk of human error.
Different samples enter the circuit at different points, but let’s start with a blood culture with suspected bacterial infection. The current state of the art is an automated microbial detection system that offers advantages in every dimension of blood culture testing.
These automated devices should offer space-saving modular design, touch screen operation, and flexible data management options. Ideally, automated blood culture systems should include colorimetric technology, which sounds an alarm based on the absorbance of light of a certain wavelength. Rather than visually checking sample, a recurring scan of light checks each vial and sounds an alarm when the light absorbance signature matches the signature of blood samples colony growth. These systems should be able to demonstrate unsurpassed recovery of a wide variety of organisms.
Next in the circuit is a system that includes a device, reagents, and software for microbial genotyping of bacteria and fungi. This composite set of tools provides a powerful method for tracking the spread and source of microbial infection, contamination, and epidemics—both natural and human-made. These systems, when equipped with rep-PCR technology, allow the lab to quickly and accurately distinguish at the subspecies and strain level.
Automated gram-stainer technology has improved dramatically in recent years. These devices now work for all types of specimens and provide accurate, standardized results. Some daunting challenges for this automation technology, including standardized coloration and eliminating cross-contamination, have been solved.
Similarly, automated plate streaking is arguably superior to manual plate streaking. The manual method of isolating bacterial strains from a sample is called the streak plate procedure. This is performed when a laboratorian spreads bacteria from a sample onto the agar within a culture plate using a wire loop. The process involves creating a zigzagging pattern on the agar in order to spread bacteria and foster growth of individual populations of bacteria.
If done properly, the pockets of bacterial colonies will thrive with enough space in between them. However, humans make mistakes, and an eight-hour shift of plate streaking inevitably leads to poorly streaked plates. Automation is a perfect match for this problem. The goal of automating a process is to improve it by freeing staff from repetitive and tedious tasks—the very kind of tasks with high error rates.
The microbiology pathway is rife with these kinds of tasks. Automating the plate streaking process proved more difficult than many thought it would be. Replicating the human plate streaking “signature” didn’t work. Instead, the solution came in the form of a rather simple design: a comb. The comb applicator uses high precision technology standardizes and maximizes colony inoculation. This device provides pre-analytical microbiology automation, automated microbiology specimen processing, improved efficiency, workflow and standardization enhancements, and improved downstream productivity with more isolated colonies.2
The microbiology lab of the future
As far as automation and process improvement have come in the microbiology lab, the future holds even greater potential. Technologies are being developed that won’t simply automate what humans currently do. These technologies will actually change the science fundamentally.
One example is mass spectrometry. This is an old technology with a very new and revolutionary potential for the microbiology lab. In the same way mass spec analyzes compounds and determines their molecular structures by “weighing” the mass of compounds and comparing the unique signature of each compound to the known qualities of the elements, a technique called Matrix Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF-MS) does the same for microorganisms.
Another technology being developed is intelligent incubation. Once plates are streaked by the automated plate streaker, the technology exists to automatically feed these plates via conveyer belt to an incubator. At given intervals, the plates flow from the incubator to an optical scanner, which can detect growth at the most nascent point—growth the human eye cannot detect. If there is no growth, plates can be sent back to the incubator and rescanned several hours later. If there is growth, an automated colony “picker” will remove the growth and send it for antibiotic susceptibility testing.
This technology isn’t available yet, but it is inevitable considering the alternative. The analytical phase is currently very hands-on and prone to waste and error. Staff spend hours shuffling plates in and out of incubators. Depending upon the size of the lab, this may only occur once a day—so visible growth goes unnoticed for an entire day. Imagine the benefits to patient care if growth were automatically detected and reported on an ongoing basis.
Automation is also poised to change the blood culturing process and virtually remove human involvement. Systems under development provide intuitive software with touchscreen design, automated loading and unloading, conveyor technology, and integrated barcode scanning. The software sends positives for identification and removes negatives immediately to speed throughput.
The microbiology pathway: samples to results
Some of the technology platforms could be game-changers in terms of providing high-quality, extremely rapid results to physicians for actionable decisions for their patients. There is ample data that the efficient, nimble, and results-focused lab can dramatically improve patient outcomes.
In studies conducted at the Southern Illinois University School of Medicine and Memorial Medical Center, researchers compared standard detection methods for respiratory viral infections to a rapid system optimized to provide fast results using the VITEK antibiotic susceptibility platform. By providing this data to physicians more quickly, the rapid system saved money, reduced length of stay, and saved lives.3,4
Information is the key to the future of healthcare, and the microbiology lab is a font of incalculably valuable information. Speeding the delivery of this information is crucial; automation and process improvement can do this. Provider reimbursement, financial incentives (and fines), and preferential care-provider status all hinge on information. Hospitals are being monitored for practicing evidence-based medicine, using data efficiently, reducing waste, and improving care. The modern microbiology lab will play a vital role in this new landscape.
References
- Campos J. Lean lab in action. MLO. 2012;44(3):26-29.
- Glasson JH. Evaluation of an automated instrument for inoculating and spreading samples onto agar plates. J Clin Microbiol. 2008;46(4):1281-1284.
- Wang, YF. The lab’s increasing arsenal of tools to battle antibiotic resistance. MLO. 2011;43(11):41.
- Barenfanger J, Drake C, Leon N, Mueller T, Troutt T. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J Clin Microbiol. 2000;(38)8:2824-2828.
About the Author

Nedal Safwat, PhD
is QIAGEN’s Vice President of Sales for North America Molecular Diagnostics. He is focused on delivering to customers leading technologies, workflows and supporting systems to accurately identify the building blocks of life in every patient sample. He brings significant experience in the IVD market and the life science industry through a variety of roles including leading a business franchise, global marketing, product management, and commercial positions. Dr. Safwat earned his doctoral and undergrad degrees in biochemistry from North Carolina State University.