Measuring protein tumor markers outside the primary tissue in breast carcinoma
A diagnosis of breast cancer strikes fear in women of all ages. In the United States, breast cancer is the most commonly diagnosed cancer for women. While early detection and treatment for breast cancer have progressed during the last decade, the disease continues to be the second-leading cause of cancer death in women of all ages and races. Each year approximately 210,200 women are diagnosed with breast cancer, and more than 40,000 of them die from the disease annually in the United States.1
CONTINUING EDUCATION
To earn CEUs, visit MLO’s CE page.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
- Discuss various features of cancer such as incidence, metastasis, and laboratory determination, including immunohistochemistry.
- Explain the various aspects of Ki-antigen, including its use for detection in breast cancer progression from tumor to lympth node metastasis and survival.
- List and explain in detail the three areas of profound changes that relate to transformation in imaging including genomic testing, business application, and digital imaging as a workflow solution in the lab.
More frequently during the last 10 years, pathologists, radiologists, clinical scientists, and clinical physicians have united in treating breast cancer patients and in researching the disease. While many avenues exist to research breast cancer, one promising method is to determine which tumors will proliferate and which ones will grow slowly.
A recent study found that by measuring the levels of the Ki-67 protein in tumors that have spread beyond the breast into the lymph nodes, pathologists can much more accurately predict whether a patient’s breast cancer is fast- or slow-growing. This finding differs from information derived only from measuring the Ki-67 levels in the primary tumor of the breast.
Several past studies2-7 have proven the prognostic significance of cell proliferation in breast cancer and its positive relationship with tumor grade, size, mitotic activity, hormonal, and human epidermal growth factor receptor 2 (HER2) status, and tumor progression. The Ki-67 antigen is expressed in cycling cells, providing an accurate measure of the growth fraction of a tumor.
Assessing the Ki-67 antigen through immunohistochemistry, using the MIB-1 antibody, signifies a simple and dependable method of predicting cell proliferation. The Ki-67 antigen continually undergoes chemical, physical, or biologic change. Additionally, it is a nonhistone nuclear protein, which is tightly linked to the cell cycle and is expressed in mid G1, S, G2, and M phases of proliferating cells but not in quiescent or resting cells of the G0 and early G1 phases.8-10
This antigen may serve as a biomarker to indicate the growth factor of a given cancer cell population. Direct correlation between Ki-67 expression in breast cancer cells and prognosis for disease-free survival and overall survival has been reported.11, 12
The multidisciplinary scientific team
A team of scientists investigated the prognostic significance of Ki-67 expression in axillary lymph node metastases as compared to the matched primary tumor. I was privileged to work with other clinical scientists involved in this research study: Fang Fan, MD, PhD, FASCP; Kareem Tawfik, MD; Bruce F. Kimler, PhD; and Marilyn K. Davis, SCT(ASCP).
Our team studied 103 breast carcinomas in patients with regional lymph node metastasis tumors at the University of Kansas Medical Center and presented the findings through an abstract and poster at the 2012 American Society for Clinical Pathology Annual Meeting held from Oct. 31 through Nov. 3, 2012, in Boston.
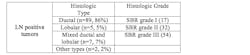
In this study, the patients had varying grades of breast cancer. Seventeen women had Scarff-Bloom-Richardson (SBR) grade I; 32 had SBR grade II; and 54 had SBR grade III13-16 and were evaluated using the Automated Cellular Imaging System (ACIS) for Ki-67 expression in primary tumor and lymph nodes. Histologic types included 86% ductal, 5% lobular, 7% mixed ductal and lobular, and 2% other types.
The samples came from either lumpectomy or mastectomy specimens of patients who ranged in age from 24 to 82 years with a mean age of 54.5 plus 13.3 years. Forty-two patients were 50 years old or younger, and 61 patients were over 50. At the time of the study, 85 patients were alive, and 18 had died from breast cancer. Histopathologic parameters, including histologic grade, type, angiolymphatic invasion, and lymph nodes status were recorded for all patients. All tumors were graded using the modified “Nottingham” criteria of Bloom and Richardson.16 The mean primary tumor size for the entire group was 3.3 ± 2.4 (SD).
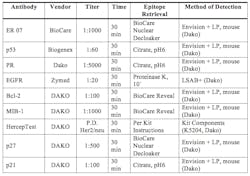
Tissue blocks containing the most representative and well-preserved tumor areas were selected for immunohistochemistry. MIB-1, estrogen receptor (ER), progesterone receptor (PR), p53, epidermal growth factor receptor (EGFR), B-cell lymphoma 2 (Bcl-2), and HER2 immunohistochemistry analyses were performed on tissue fixed with 10% neutral buffered formalin, using a DAKO autostainer. The paraffin-embedded tissue blocks were cut to 5-micrometer sections, deparaffinized, and heat-treated for antigen retrieval. HER2 antibody was detected using the HercepTest (DAKO), per the manufacturer’s protocol.
For individual antibodies, the vendor, titration titer, time of titration, epitope retrieval method, and method of detection, please refer to Table 1. Hematoxylin was used as a counterstain. Appropriate positive and negative controls were included.
Table 1. Protocols for Immunohistochemistry
Positive controls for the markers were selected from surgical specimens received in the surgical pathology laboratory and confirmed to be positive when compared with other samples. Negative controls included samples run without the primary antibody or with nonimmune serum. Positive and negative controls supplied in the HercepTest kit were used for the evaluation of the HER2 stains. Nuclear morphology, tubular formation, and MIB-1 labeling index were then analyzed and assigned scores.
The power of immunohistochemistry
Positive immunohistochemistry reactions were defined as a dark brown circumferential reaction on the cell membrane for HER2 and EGFR, distinct nuclear staining for MIB-1, ER, PR, and p53, and intense cytoplasmic staining for Bcl-2. Staining parameters were evaluated at 10x, and areas of high-density immunostaining were chosen for image analysis or manual scoring. For proliferation index (PI) of MIB-1, the percentage of nuclei with immunopositivity was determined using the PI program of either the Cell Analysis System (CAS) 200 image analyzer (Bacus Laboratory) for the period from 1991 to 2001 or later with the Clarient Automated Cellular Imaging System.
Five to 10 areas with the highest staining intensity were selected for quantification from each specific lesion. An average score for all selected areas was then calculated.
For ER, PR, and p53, both the CAS-200 and ACIS systems were used for automated counts. Manual microscopy was utilized to score tumor staining with antibodies to EGFR and Bcl-2. HER2 staining was quantified using a score of 0 or 1+ to indicate “negative” results, and 2+ or 3+ to represent “positive” results, per the scoring instructions included in the HercepTest kit. The results were validated using the HER2 scoring system of the ACIS machine.
Using both manual and automated microscopy, up to 10 high power fields were evaluated with each marker to provide the final score. A staining of 10% or more of the tumor cells with the antibody to EGFR or Bcl-2 was considered positive, whereas for p53, any counts greater than or equal to 5% were considered positive.
The battle with metamorphosis in tumors
Overall, frequencies and percentages were summarized for tumor grade, ER, PR, p53, EGFR, Bcl-2, HER2, vascular invasion, and node positivity. The frequencies of each variable stratified by the grading system were calculated, and their relationships to each grading system were evaluated using the chi-square test. The Log-rank test was used to compare overall survival across the different groups independently.
Ki-67 (MIB-1) percent expression was compared on the sample specimens between primary tumor and lymph nodes, and correlated with age, grade, ER, PR, p53, EGFR, Bcl-2, HER2 status, and the overall survival of these patients. The results showed that measurement of Ki-67 in lymph nodes is not only valuable but may be superior to the evaluation of Ki-67 expression in the primary tumor for predicting overall survival of patients with metastatic breast cancer.
The study discovered that Ki-67 antigens have two ways of spreading in the lymph nodes. One remains at a low proliferation. The second type spreads rapidly through high proliferation, becoming aggressive outside of the breast tissue. In fact, its characteristics change like Dr. Jekyll morphing into Mr. Hyde, and become a completely different entity. The patients with the aggressive, altered Ki-67 have worse outcomes than patients with the low proliferation type of Ki-67.
Pathologists have suspected that not all tumors are created equal, and now they have the scientific correlation. This additional knowledge can directly improve patient outcomes through more informed therapeutic options. The pathologist can advise an individual patient’s surgeon and clinician about which subtype of Ki-67 antigen was found in the lymph nodes. Based on that information, some patients may require additional chemotherapy and/or radiation to recover their health. Other patients will benefit from less aggressive treatment.
All types of cancer, including breast cancer, are wily biologic enemies of good health. Tragically, cancer cells are smart. They can metamorphose, evade a patient’s immune system, and fool pathologists, laboratory professionals, and clinicians. This study gives us another weapon to use against the spread of breast cancer for individual patients and points scientists in the right direction.
Summary
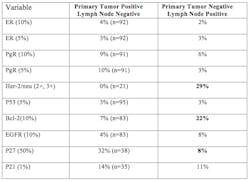
This study found a positive correlation between the incidence of lymph node metastasis and primary tumor size, histologic grade, type, age, p53, HER2, and Ki-67 status and a negative correlation with ER/PR status. When primary tumors are compared with their corresponding metastatic lesions, there are no biomarker differences between them.
Low Ki-67 (MIB-1) index in lymph node metastatic tumors, but not for primary tumors, is a favorable prognostic factor. Using a cutoff of 10%, there is no difference in overall survival for patients with primary tumors less than, equal to, or greater than 10% Ki-67. In contrast, patients with lymph node metastatic tumors with less than 10% Ki-67 had significantly longer survival than those with greater than 10% Ki-67.
Measuring proliferative activity of metastatic breast carcinoma to regional lymph nodes demonstrated definite value as a prognostic factor. This study’s results suggest that patients with higher proliferative activity in lymph node metastasis may require more aggressive therapy and a closer clinical monitoring of their disease.
The next step is to look at the tumor cells that proliferated from the molecular level. How can scientists stop these tumor cells from growing? Teams of scientists need to conduct follow-up research that builds on these findings and critically evaluates them before changing the standard of care for breast cancer patients.
References
- United States Cancer Statistics. 1999-2008 Cancer Incidence and Mortality Data. Web-based Report. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. http://apps.nccd.cdc.gov/uscs/. Accessed November 30, 2012.
- Bouzubar N, Walker KJ, Griffiths K, et al. Ki-67 immunostaining in primary breast cancer: pathological and clinical associations. Br J Cancer. 1989;59:943-947.
- Jalava P, Kuopio T, Juntti-Patinen L, Kotkansalo T, Kronqvist P, Collan Y. Ki-67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology. 2006;48:674-682.
- Jansen RL, Hupperets PS, Arends JW, et al. MIB-1 labeling index is an independent prognostic marker in primary breast cancer. Br J Cancer. 1998;78:460-465.
- Locker AP, Birrell K, Bell JA, et al. Ki-67 immunoreactivity in breast carcinoma: relationships to prognostic variables and short term survival. Eur J Surg Oncol. 1992;18:224-229.
- Pinder SE, Wencyk P, Sibbering DM, et al. Assessment of the new proliferation marker MIB-1 in breast carcinoma using image analysis: associations with other prognostic factors and survival. Br J Cancer. 1995;71:146-149.
- Trihia H, Murray S, Price K, et al. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors—a surrogate marker? Cancer. 2003;97:1321-1331.
- Gerdes J, Lemke H, Baisch H, Wacker H-H, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710-1715.
- Lehr HA, Hansen DA, Kussick S, et al. Assessment of proliferative activity in breast cancer: MIB-1 immunohistochemistry versus mitotic figure count. Human Pathol. 1999;30:1314-1320.
- Petit T, Wilt M, Velten M, et al. Comparative value of tumour grade, hormonal receptors, Ki-67, HER-2 and topoisomerase II alpha status as predictive markers in breast cancer patients with neoadjuvant anthracycline based chemotherapy. Eur J Cancer. 2004;40:205-211.
- Tawfik O, Kimler BF, Davis M, et al. Grading invasive ductal carcinoma of the breast: advantages of using automated proliferation index instead of mitotic count. Virchows Arch. 2007;450:627-636.
- Stasik C, Davis M, Kimler B, et al. Grading ductal carcinoma in situ of the breast using an automated proliferation index. Annals Clin Lab Sci. 2011;41:122-130.
- Scarff RW, Torloni H. Histological typing of breast tumors. In: International Histological Classification of Tumours. World Health Organization. 1968;2:13-20.
- Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer. Br J Cancer. 1957;11:359-377.
- Elston CW. The assessment of histological differentiation in breast cancer. Aust N Z J Surg. 1984;54:11-15.
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403-410.
Is measurement of Ki-67 in axillary lymph node metastasis of breast cancer useful?
As discussed in the accompanying article, the study authors believe that they have demonstrated the answer is yes. Here’s a look at some of the supporting Tables and Figures that were part of the ASCP poster presentation last fall, with relevant comments. Note: the protocols for immunohistochemistry, described in the article, are delineated in Table 1 (above). The following table and figures express the results of Dr. Tawfik and his colleagues’ research.)
Table 2. Histopathologic characteristics of invasive mammary carcinoma tumor with metastasis to regional lymph nodes.
Figure 1.
Using a cutoff of 10%, there was no difference in overall survival for patients with primary tumors < = or > than 10% Ki-67. In contrast, patients with lymph node metastatic tumors with >10% Ki 67 had significantly longer overall survival than those with <10% Ki 67.
Figure 2.
Primary tumors with <10% Ki-67 proliferative indices had a favorable survival when their corresponding LN metastases remained at <10% Ki-67 indices as compared to those showing >10% proliferative activity.
Figure 3.
Additionally, the majority of primary tumors with >10% Ki-67 proliferative indices, had worse survival when their corresponding LN tumors remained with >10% Ki-67 indices. In contrast a smaller group of patients with nodal metastasis having <10% Ki-67 proliferative activity had a much more favorable outcome.