All of us have experienced the familiar symptoms of gastrointestinal (GI) distress—bloating, cramping, abdominal distention, and pain. Most of us reach for an antacid, resolve to make smarter food choices, and hope to feel better soon. Not so for the 25% of Americans who suffer from adverse food reactions that lead to prolonged GI distress.1 More than 3.6 million of them visit their primary care doctors seeking relief from digestive problems each year.2 Potential causes range from acute GI infections and food intolerances or sensitivities to such serious conditions as life-threatening food allergy and celiac disease. Diverse and nonspecific GI symptoms seldom raise the suspicion of autoimmune etiology. Indeed, patients with celiac disease wait an average of 11 years before a correct diagnosis is made, and one-third of them have previously been diagnosed with irritable bowel syndrome.3 Today, readily available serologic tests can clarify the diagnosis of GI distress much sooner, helping to avoid the long-term complications of untreated autoimmune disorders such as celiac disease.
Guideline-based diagnosis
Patients experiencing GI symptoms usually turn to over-the-counter remedies first, experimenting with antacids or heartburn medications. Should those fail, a visit to the primary care physician typically results in a trial of medication and perhaps advice regarding dietary choices. Diagnostic testing is generally performed only if symptoms persist. Typical first-line testing for persistent GI symptoms might include a complete blood count, C-reactive protein or erythrocyte sedimentation rate (as measures of inflammation), CHEM-18 (for electrolytes), and stool studies. Often these tests do not identify a cause for the symptoms, and at that point a work-up for food allergy is in order. Approximately 2% to 4% of adults and 4% to 8% of children have food allergies.4
The National Institutes of Health Guidelines for the Diagnosis and Management of Food Allergy (2010) recommend specific IgE (s-IgE) testing, especially for common allergenic foods such as milk, egg, wheat, peanut, soy, fish, tree nuts, and shellfish.5 A positive s-IgE result signals IgE sensitization and should be combined with the patient’s history to make an accurate diagnosis of food allergy. It should be noted that both IgE sensitization and manifest clinical symptoms are necessary for a diagnosis of food allergy, as sensitization can exist in the absence of clinical symptoms. A double-blind, placebo-controlled oral food challenge is considered the gold standard for confirming a diagnosis of food allergy. However, these are time-consuming, poorly reimbursed, and may pose a risk of anaphylaxis.6 Treatment of a food allergy consists of eliminating offending foods from the diet. It may also be appropriate to prescribe an epinephrine injector.
Among the reasons for a long delay in diagnosing celiac disease are the assumption that it is rare and the wish to avoid intestinal biopsy unless absolutely necessary, especially in young children. In fact, epidemiologic studies demonstrate that 0.5% to 1% of the population in Western Europe and North America are affected.7 Thus the diagnosis may be missed in hundreds of thousands of people who have no symptoms or only vague complaints of GI distress. Onset of celiac disease typically occurs either between the ages of 6 months and 2 years, after gluten has been introduced into the diet, or between ages 20 and 40 years. In 73% of cases the first clinical signs appear before one year of age. Clinical presentations differ somewhat according to age (Table 1). Asymptomatic or minimally symptomatic forms of celiac disease are much more frequent than overt symptoms. Individuals who are HLA-DQ2 or HLA-DQ8 positive exhibit a strong genetic susceptibility for celiac disease. In addition, it sometimes accompanies other organ-specific autoimmune diseases, such as insulin-dependent diabetes, autoimmune thyroiditis, or primary biliary cirrhosis, or systemic connective tissue diseases such as lupus or Sjögren’s syndrome.
The traditional diagnosis of celiac disease depends on confirmation of villous atrophy, cryptic hyperplasia, or intraepithelial lymphocytosis in samples collected during small intestinal biopsy.7 Serologic screening tests were developed because biopsy is invasive, expensive, and unpleasant and carries some risk.8 Therefore, current European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines (2011) recommend serologic testing measuring recombinant human tissue transglutaminase (rhtTG) IgA antibodies before biopsy.9 Diagnostic serology for celiac disease should only be performed before gluten is eliminated from the diet. Otherwise, false negative results are likely.
Treatment for celiac disease consists of supplementation for nutrient deficiencies or conditions (such as anemia) resulting from malabsorption and adherence to a gluten-free diet. Corn, rice, and potatoes are allowed, while wheat, rye, and barley-containing products must be avoided. Processed foods often contain gluten, so a strict gluten-free diet can be difficult to maintain for a lifetime.
Serologic testing options
The first serologic IgA screening test for celiac disease, the endomysial antibody or EMA assay, debuted in 1983. The test uses indirect immunofluorescence microscopy to analyze sections of monkey esophagus that serve as the antibody substrate.10 In one systematic review, the pooled sensitivity and specificity of EMA were 94% and 98% respectively.11 The EMA rapidly became the standard in serologic testing for celiac disease, despite its being subjective, labor-intensive, and reliant on tissue from an endangered species.8 The antigen recognized by EMA was eventually identified in 1997 as tissue transglutaminase (tTG). A new enzyme-linked immunosorbent assay (ELISA) using guinea pig liver antigen tTG (gptTG) soon followed, as did the purified human erythrocyte (htTG) and recombinant human (rhtTG) versions. These tests are fully quantitative, highly automated, and widely available to primary care physicians.
The EMA and ELISA tests have been compared in a number of peer-reviewed papers. For example, Lewis and Scott examined 34 controlled trials of serologic IgA diagnostic tests for celiac disease, including EMA and gptTG and rhtTG.8 In head-to-head comparisons between EMA and tTG, EMA was more likely to perform better for both sensitivity and specificity. However, excluding gptTG tests demonstrated that rhtTG tests were more sensitive than EMA, though not more specific. The sensitivity tended to be higher in adults than in children. The authors concluded that the rhtTG tests performed much better than the gptTG tests, and called rhtTG the preferred test for screening asymptomatic people and excluding celiac disease when its pre-test probability was low (<25%). In the case of rhtTG-positive results, they recommended a small bowel biopsy to confirm the diagnosis.
Wong, et al compared 13 ELISA kits and concurred with Lewis and Scott regarding the superiority of rhtTG to gptTG.12 They found that one rhtTG assay (Varelisa/Pharmacia & Upjohn Diagnostics, Germany) achieved the same 100% sensitivity and specificity as EMA. The antigen for that particular assay uses human recombinant tTG expressed in insect cells as antigen (Baculovirus/Sf9 system) to ensure its purity. As recommended by the manufacturer, cut-off values and decision thresholds determined by receiver operating characteristic (ROC) plots should be used to calculate sensitivity and specificity. The authors emphasized that laboratories should consider the source of tTG antigen but also the performance of the test using locally derived cut-off values when selecting an IgA ELISA kit.
An audit of routine laboratory practice sheds additional light on the diagnostic accuracy of tTG ELISA as a first-line diagnostic test for celiac disease.13 The specificity was determined using 1554 routine celiac disease serologic samples from adults. Sensitivity was determined from 75 consecutive new adult diagnoses of celiac disease. The prevalence of new diagnoses in the tested population was 2.8%. Specificity with a cut-off value of 3 units/mL was 98.9%. Sensitivity of the rhtTG ELISA was 92%, identical to that of EMA. The authors noted that IgA deficiency should be excluded in the presence of low absorbance values in the tTG assay. Such a deficiency is 10 to 15 times more common in patients with celiac disease than in healthy subjects. Anti-tissue transglutaminase IgA assays which produce results with no detectable IgA antibody may be indicative of a patient with selective IgA deficiency, and these patients should be further investigated.14 Patients with an IgA deficiency should be screened for celiac disease with a reliable anti-tTG IgG assay.7 When diagnosing celiac disease, anti-tTG IgA testing is more specific but less sensitive, whereas IgG is more sensitive but less specific. Consequently, testing for both isotypes is usually recommended.
Laboratory directors can assist primary care physicians by explaining the nuances of the diagnostic tests for celiac disease. Many laboratories follow a step-wise algorithm, beginning with s-IgE testing for food allergies, and continuing to IgA rhtTG testing if celiac disease is suspected. A pre-selected profile of four key celiac disease biomarkers simplifies test ordering for primary care physicians. If IgA deficiency seems likely, the physician can be directed to IgG testing.
Is biopsy always necessary?
Extensive experience with EMA, rhtTG, and biopsy in the diagnosis of celiac disease has led to refinements in their clinical application. A recent study investigated the possibility that biopsy might be avoided when diagnosing celiac disease in pediatric patients from the ages of 0.7 to 17.8 years.15 The authors retrospectively examined histological slides and serology results from 283 pediatric patients who were suspected of having celiac disease. In 128 of the patients the IgA rhtTG level was ≥ 100 U/mL, and 124 of them had histologic evidence that confirmed a diagnosis of celiac disease. Only one of the 128 patients with rhtTG ≥ 100 U/mL had no histologic evidence of abnormality. False-positive results occurred in 34.2% of EMA tests and 17.5% of rhtTG tests; false-negative results occurred in 3.7% of EMA tests and 4.3% of rhtTG tests. Both tests had a sensitivity of 96%, but specificity for rhtTG was 83% compared to values as low as 66% for EMA. The positive predictive value for EMA was 79% compared to 88% for rhtTG. Specificity is particularly important in diagnosing celiac disease, given the fact that even a slight decrease in specificity results in a dramatic increase in unnecessary intestinal biopsies.
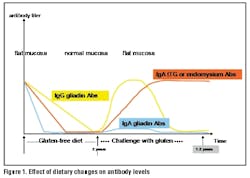
On the basis of this study data, the authors believe that a gluten-free diet should be started in all pediatric patients with a rhtTG level ≥ 100 U/mL and that biopsy can be avoided if symptoms abate.15 Tissue transglutaminase or EMA antibodies will steadily decrease after six to 18 months on a gluten-free diet, eventually disappearing altogether.7 Repeat serologic testing is recommended six to 12 months later as a means of monitoring compliance with the diet. Demonstrating reduced IgA antibody levels may help motivate patients or caregivers to continue with the dietary restrictions (Figure 1).
Another retrospective, controlled study suggests a different diagnostic strategy for celiac disease in children younger than 18 months of age.16 Antibodies against gliadin IgA (AGA-IgA), tTG-IgA, and EMA-IgA were analyzed for 428 children with biopsy-confirmed celiac disease and for 216 controls. Gliadin, a glycoprotein present in wheat, participates in gluten formation. In children younger than 18 months, the sensitivity of AGA-IgA testing was significantly better (97%, P<0.0001) than that of tTG-IgA and EMA-IgA (both 83%). This finding led the authors to propose testing for both AGA-IgA and tTG-IgA when selecting children younger than 18 months for biopsy to confirm celiac disease. However, in children older than 18 months, they suggest that IgA tTG or EMA can be used alone.
Improved specificity with deamidated gliadin peptides
Recent research supports the value of using deamidated gliadin peptides (DGP) in AGA diagnostic testing.17 Gliadin peptides that cross the mucosal border in patients with celiac disease are deamidated by tTG, making them much more immunogenic than unprocessed gliadin. As a result, deamidated gliadin represents a more specific target for the gliadin antibodies produced in these patients. Using relevant synthetic DGPA in an assay improves specificity over native gliadin antibodies, as illustrated in two studies.
The first study tested patients newly diagnosed with celiac disease before and after starting a gluten-free diet, patients with persistent small bowel villous atrophy despite a strict gluten-free diet, and patients reporting abdominal symptoms after eating cereal.18 The results from EMA, conventional AGA, tTG and DGP-AGA were then compared. The DGP-AGA and tTG-IgA tests performed equally well, with a sensitivity of 91% and a specificity of 98% in celiac disease. The specificity of EMA-IgA was also high, but its sensitivity was only 80%. The conventional AGA had both poor sensitivity and specificity. The best sensitivity without loss of specificity was obtained by using both the DGP-AGA and tTG-IgA assays.
The second study compared four commercially available IgG anti-DGP assays for diagnostic accuracy (sensitivity and specificity) against AGA and anti-tTG assays in 86 consecutive patients with celiac disease and 741 control subjects.19 At a specificity of 98%, the anti-DGP assays’ sensitivities varied from 76.7% to 86.0%. Specificity ranged from 97.3% to 99.3%. The specificity of the IgA and IgG anti-gliadin assays was significantly lower than that of the IgG anti-DGP assays (P<0.05). This study was the first to demonstrate that IgG anti-DGP assays performed as well as tTG-IgA assays for the diagnosis of celiac disease. The good specificity of the IgG-DGP assay suggests it could be used in combination with the tTG-IgA testing to replace total IgA measurements as a means of identifying patients who are IgA deficient.
The increasing clinical value of autoimmune biomarkers
Diagnosing autoimmune diseases such as celiac disease has never been easy. The presence of autoimmune antibodies serves as the basis for reliable diagnostic tests that measure specific biomarkers associated with certain diseases. Measuring tTG IgA and IgG, combined with DGP IgA and IgG, provides a practical initial testing approach for celiac disease in the primary care setting. DGP IgA and IgG antibodies are excellent markers for celiac disease and necessary to support positive tTG-IgA results. The wide availability of automated IgG and IgA assays allows primary care physicians to work with immunologists in diagnosing, treating, and monitoring the progress of patients with celiac disease. Similar diagnostic advances in serologic IgE testing have changed practice patterns in the diagnosis and treatment of airborne allergies and asthma, which are increasingly being managed in primary care.20 Offering physicians rhtTG testing helps clarify the etiology of recurrent GI distress and channel patients to appropriate and timely treatment.
References
- American College of Allergy, Asthma & Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006;96(3 suppl 2):S1-S68.
- Middleton K, Hing E, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 Outpatient Department Summary. Advance Data. 2007;389:14.
- Green PHR, Stavropoulos SN, Panagi SG, et al. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96(1):126-131.
- American Academy of Allergy Asthma & Immunology. Allergy statistics. http:/www.aaaai.org/about-the-aaaai/newsroom/allergy-statistics.aspx. Accessed October 8, 2012.
- National Institute of Allergy and Infectious Diseases. Guidelines for the Diagnosis and Management of Food Allergy in the United States. Summary of the NIAID-Sponsored Expert Panel Report. Bethesda, MD: National Institutes of Health; December 2010. NIH publication 11-7700.
- Pongracic JA, et al. Oral food challenge practices among allergists in the United States [Letters to the Editor]. J Allergy Clin Immunol. 2012;126(2):564-566.
- Olsson NO, Musset L, Chyderiotis G, et al. Celiac disease. In: Shoenfeld Y and Meroni PL, eds. The General Practice Guide to Autoimmune Diseases. Miami: Pabst Science Publishers; 2012:193-201.
- Lewis NR, Scott BB. Systematic review: the use of serology to exclude or diagnose coeliac disease (a comparison of the endomysial and tissue transglutanimase antibody tests). Aliment Pharmacol Ther. 2006;24:47-54.
- Husby S, Koletzko S, Korponay-Szabó IR, et al. The ESPGHAN Working Group on Coeliac Disease Diagnosis 2011. ESPGHAN Guidelines for the diagnosis of coeliac disease in children and adolescents: an evidence-based approach. J Pediatr Gastroenterol Nutr. 2012; in press.
- Bűrgen-Wolff A, Dahlbom I, Hadziselimovic F, Petersson CJ. Antibodies against human tissue transglutaminase and endomysium in diagnosing and monitoring coeliac disease. Scand J Gastroenterol. 2002;(6):685-691.
- James MW, Scott BB. Endomysial antibody in the diagnosis and management of coeliac disease. Postgrad Med J. 2000;76:466-468.
- Wong CW, Wilson RJ, Steele RH, et al. A comparison of 13 guinea pig and human anti-tissue transglutaminase antibody ELISA kits. J Clin Pathol. 2002;55:488-494.
- Hills PG, Forsyth JM, Semeraro D, Holmes GKT. IgA antibodies to human tissue transglutaminase: audit of routine practice confirms high diagnostic accuracy. Scand J Gastroenterol. 2004;11:1078-1082.
- Lőwbeer C. Undetectable anti-tissue transglutaminase IgA antibody measured with EliA [letter to the editor]. Clinica Chimica Acta. 2012; in press.
- Mubarak A, Wolters VM, Gerritsen AM, et al. A biopsy is not always necessary to diagnose celiac disease. JPGN. 2011;52(5):554-557.
- Lagerqvist C, Dahlbom I, Hansson T, et al. Antigliadin immunoglobulin A best in finding celiac disease in children younger than 18 months of age. JPGN. 2008;47:428-435.
- Sugai E, Vazquez H, Nachman F, et al. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin Gastroenterol Hepatol. 2006;4:1112-1117.
- Kaukinen K, Collin P, Laurila K, et al. Resurrection of gliadin antibodies in coeliac disease. Deamidated gliadin peptide antibody test provides additional diagnostic benefit. Scand J Gastreoenterol. 2007;42:1428-1433.
- Vermeersch P, Ceboes K, Mariën G, et al. Diagnostic performance of IgG anti-deamidated gliadin peptide antibody assays is comparable to IgA ani-tTG in celiac disease. Clinica Chimica Acta. 2010;411:931-935.
- Staples S. Advances in allergy testing: results from the largest allergy study. Clin Lab Prod. 2012;March:8-12.