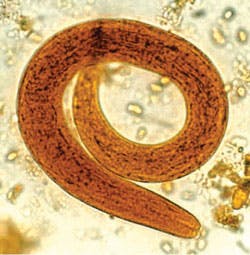
threadworms, is a nematode helminth parasite that causes
strongyloidiasis.
A 78-year-old male
followed up with his physician for symptoms of chronic weight loss and
loose stools. The patient was previously diagnosed with hypertension,
chronic renal disease, heart disease, erectile dysfunction, and chronic
alcohol abuse. He was suffering from anemia from a mitral valve repair.
Elevated creatinine levels and a low estimated glomerular filtration
rate, or GFR, indicating kidney problems were observed. The patient
stated he thought his prescribed medication was curbing his appetite and
causing his apparent weight loss and loose stools. He, therefore,
discontinued taking his medications for duration of a year. The
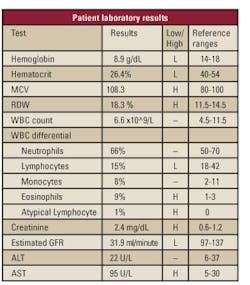
patient's laboratory results (see Table 1) exemplified the constancy of
his underlying conditions but notably revealed a high eosinophil count
of 9%, a possible indication of a parasitic infection.
A stool specimen was sent to the microbiology
laboratory for routine culture, and ova and parasite (O&P) examination. The
stool specimen visually resembled rice water stool, a condition consistent
with the gastrointestinal pathogen Vibrio cholerae. O&P
examination was performed on the day of its arrival, and a visual assessment
of the larvae morphologically confirmed the diagnosis of Strongyloides
stercoralis. Distinguishing morphological characteristics of the larvae
were consistent with the rhabditiform larvae stage of the parasite.
A hyperinfection could not be determined because of
the extreme liquid state of the stool specimen. Thus, the specimen was
centrifuged to concentrate the stool in order to determine the severity of
the infection. The findings determined that the patient suffered from an
acute manifestation of S stercoralis. There was a speculation that
this patient's infection could have exceeded 20 years because of his past
travels to Southeast Asia. Interestingly, the longest documentation of
infection with S stercoralis is 65 years.1
Epidemiology and historical aspects
S stercoralis, also called threadworms, is a
nematode helminth parasite that causes strongyloidiasis.2 There
are an estimated 100 million to 200 million people infected with S
stercoralis
residing in 70 different countries.1,3,4 The true prevalence of
an S stercoralis infection is underestimated because a majority of
the cases are sub-clinical.1 There are 53 species of the
organism, and the most common infection is due to the species S
stercoralis. Species such as S fuelleborni and S kellyi
are frequently found in humans living in Africa and Papua New Guinea.1
S stercoraliswas first revealed in the feces
of French soldiers in 1876.3 The soldiers returned from Indochina
(Vietnam, Cambodia, and Laos) with severe diarrhea.3 The life
cycle and pathogenesis were not discovered until the early 1900s. S
stercoralis is most prevalent in warm climates but has the ability to
survive in colder climates.1 There is a high prevalence of S
stercoralis in Brazil, Central America, and Australia. It is endemic in
Africa, South and Southeast Asia, South America, rural parts of Italy, Papua
New Guinea, and the Pacific Islands such as Fiji.1-3 Endemic
areas in the United States of America are Kentucky, West Virginia, and
eastern Tennessee.1,3 A recent study illustrated that 38% of
Southeast Asian immigrants residing in Washington, DC, were infected with
S stercoralis.1 Knowledge of the geographic distribution of
S stercoralis is only significant to those who travel to endemic
areas and those who are/become infected with S stercoralis.
immigrants residing in Washington, DC, were infected with S stercoralis.
Life cycle of S stercoralis
S stercoralis'life cycle is one of the most
complicated and pathogenic life cycles in human parasitic infections. Most
cases of human infection are acquired from penetration of the skin by the
filariform larvae stage from soil.1,2,3,4,6 In rare cases, human
infection can result from ingesting contaminated foods. The filariform
larvae stage is known as the parasitic stage. The larvae are attracted to
certain chemical compounds found on human skin. One such compound is
urocanic acid found on the epidermis layer of human skin and more prevalent
when sweating. Notably, urocanic acid is five times greater on human feet
than any other skin portion on the human body. After the filariform larvae
penetrate the skin, they travel to the cutaneous blood or lymphatic vessels.1,3,4
They migrate through vessels to the lungs and travel to the pulmonary
capillaries to the alveoli.1,3 The larvae migrate up the
respiratory tree to the trachea and pharynx.1,3 It is at this
stage that the host must swallow the larvae in order for the larvae to enter
into the mucosa in the duodenum and upper jejunum.
Within two weeks, the larvae mature into only the
female form.1 Unique only to this species, the female larvae
reproduce without a male through an asexual reproductive process called
parthenogenesis.1,3 Each adult female can live for five years and
solitarily reproduce.1,3 In the next stage, the young larvae
mature in the intestine and pass through the feces as the rhabditiform
larvae.1,3
Strongyloides species are the only helminth that
secretes larvae instead of eggs in the feces. Larvae can appear in the feces
in a period as short as one month after skin penetration.1,3 In
another life cycle specific to temperate climates, larvae can free-live in
soil and reproduce as male and female to make new generations of the
parasitic filariform that is ready to infect a host.1,3 The
rhabditiform larvae, however, can also molt into the filariform larvae
within the intestines and re-infect the host. This is called autoreinfection
and usually results in a hyperinfection.1,3
Morphology
Identification of S stercoralis mostly focuses
on the active feeding stage rhabditiform due to the nature of the parasite's
life cycle.1,2 The rhabditiform larvae are long and slender, and
can grow up to 630 nm in length and 16 nm in width. They have a short buccal
cavity with a long slender esophagus and have prominent genital primordium.
The filariform larvae are the non-feeding stage; they have a longer
esophagus and more of a notched tail than the rhabditiform larvae stage.4
Usually, only the females are found in the intestinal track because of
the asexual reproduction, therefore, the morphology of male larvae are not
significant in a clinical setting.
Pathogenesis
S stercoralisinfection is mostly asymptomatic
and can remain undetected for several decades.1,2 Infections are
mostly acquired in patients who travel to endemic areas. Symptomatic
infections appear in the gastrointestinal, pulmonary, and cutaneous areas.1
The most common symptoms are anorexia, nausea, diarrhea, abdominal bloating,
abdominal discomfort, ulcers, cough, dyspnoea, wheezing, acute pulmonary
insufficiency, low-grade fever, rashes, tissue damage, and sepsis.1-3
An unusual characteristic of the parasite is
autoreinfection. It occurs when the rhabditiform larvae molts back to the
filariform and exits the intestinal mucosa, traveling through the host's
body to the lungs to start a new life cycle. Autoreinfection can occur from
slight changes in bowel movements like constipation or diarrhea. It can also
occur in patients who are immunosuppressed or taking immunosuppressive drugs
such as corticosteriods.2 Corticosteriods increase susceptibility
to infection by suppressing the immune response in eosinophils.2,3
During autoreinfection, the larvae disseminates into the intestinal track,
pulmonary tissue, and skin, resulting in a hyperinfection syndrome with a
fatality rate of 90%.1,3,4
Secondary infections associated with autoreinfection
are caused by bacteria and yeast in the normal flora of the intestinal tract
that invade sterile sites of the body.2,4 In severe conditions of
autoreinfection, sepsis is of most concern. The normal intestinal flora
adheres to the surface of the larvae, or the larvae excretes bacteria out of
alimentary canals and causes the patient to have sepsis either in the
bloodstream, tissues, organs, or lymphatics.2 Almost one-half of
patients with strongyloidiasis may develop secondary bacteria infections
with Gram-negative bacilli.3
1.
Clinical presentation
Patients with strongyloidiasis are usually
asymptomatic and cannot pinpoint the moment of contracting the parasitic
infection. Patients may show mild eosinophilia. Immunosuppressed individuals
may show no eosinophilia.2,4 If present, clinical symptoms are
not specific to strongyloidasis. In fact, an infection may mimic other
diseases such as bronchitis because of the migration of the larvae through
the lungs.2
This causes diagnosis to be difficult. Patients who have recently had their
skin penetrated by the filariform larvae may acquire an itchy cutaneous
eruption of pruritic papulovesicular lesion.1,3,4,5 The rash can
move five cm to 15 cm per hour and can appear anywhere on the body but
usually is seen on the feet. It can last from a few hours to a couple of
days.1 In autoreinfection, the migration of the parasite can last
for a few months to years in extreme cases.1
The most common reported symptoms are abdominal
bloating with pain and diarrhea that is usually not bloody.1
While breathing, patients may exhibit wheezing sounds with a slight cough.1,2
There is also a chance of hemoptysis in autoreinfected patients.
Patients are often misdiagnosed and treated symptomatically with the
parasite still inhabiting the host. This leads to chronic infections with
S stercoralis.3
Laboratory role in diagnosis
Patients with strongyloidasis are often overlooked
unless they are in a state of hyperinfection. There is 30% sensitivity that
strongyloidasis will be diagnosed from a single stool specimen. Sensitivity
increases to almost 90% if seven stool specimens are evaluated. The most
important diagnostic tool is simply collecting more stool samples over
periods of time and using a variety of different test methods to achieve
higher sensitivity in diagnosis.
It is also important to examine every field in the
microscope when performing O&P examinations because S stercoralis
larvae occur in low numbers. Identification of the parasite can be improved
if the Baermann technique is used.2 This technique involves
adding warm water to the stool and centrifuging the specimen. The parasite
is detected in the supernatant because it is attracted to warm water.
An ELISA technique is available to detect S
stercoralis IgG antibody in serum. Available methods have a sensitivity
of 88% to 95% and a specificy of 29% to 99%.2,3,6 A patient may
be positive for years after a successful treatment. Patients who are
immunosupressed may be falsely negative. This technique can cross match with
other helminth infections.2,3,6 When using this methodology for
diagnosis, it should only be used secondary to O&P examination.
Treatment of strongyloides
S stercoralishas been treated for centuries.
In the past, people used herbal substances such as papaya leaves and hog
plums to treat parasitic worm infections. Nowadays, there are two treatment
options for infections with S stercoralis, Ivermectin, and
Thiabendazole.2,3
Ivermectin, also known as Stromectol, is the drug of choice.2,3
The drug binds to the chloride ion glutamate-gated channels in the
nerve and muscle cells of the parasite. This mechanism increases
permeability to the cell membrane. Ivermectin first paralyzes the parasite,
then kills it. The cure rate is 97% within the first two days.2
It is given orally 200 mcg/kg per day in one or two doses and can also be
given through injection when patients are in a state of hyperinfection.
Thiabendazole, also known as Mintezol, is less
effective than Ivermectin.2,3 The drug is mostly used as a
general treatment for a helminth infection. It inhibits helminth-specific
mitochondrial fumarate reductase, resulting in the inhibition of a helminth
parasite's life cycle. The drug is not effective beyond the lumen of
intestines because absorption is poor and is no longer used for treatment.
Thiabendazole is given in a dosage of 50 mg/kg/day in two divided doses
given 12 hours apart for two days. If the patient is in a state of
hyperinfection, dosage is given for seven to 10 days.2,3
Case conclusion
The patient followed up with his physician about his
chronic weight loss and loose stools. The symptoms were prevalent for
roughly a year. Laboratory test results only indicated the known conditions
of hypertension, chronic renal disease, heart disease, erectile dysfunction,
and chronic alcohol abuse. The only test result with indication of his
parasitic infection was the eosinophil count of 9% (see Table 1). His stool
culture was negative for any underlining pathogens such as Salmonella,
Shigella, Campylobacter jejuni, Yersinia enterocolitica, Vibrio
cholerae, Aeromonas, and Pseudomonas aeruginosa species.
The patient's stool sample was found to be positive for S stercoralis.
There was speculation about his travels more than 20 years ago to Southeast
Asia. There was not enough evidence to prove the length of infection because
there was a possibility the infection could have been acquired in Florida.
The parasitic infection was diagnosed by visual morphology with trichrome
stain in conjunction with the patient's current symptoms. The patient was
treated with Ivermectin 200 mcg/kg in one dose.
Summary
This article illustrates a case of S stercoralis.
This organism causes the disease strongyloidasis. Strongyloidasis is defined
by acute manifestation, autoreinfection, or hyperinfection. It is contracted
by skin penetration of the filariform larvae in the soil. Diagnosis and
effective treatment is dependent on identification through special
techniques with stool samples in the clinical laboratory.
Randi Taylor, MT(ASCP), works as a
medical technologist at the Jacksonville, FL, Mayo Clinic.
Hassan Aziz, PhD, CLS (NCA), is department head of Medical Technology
at Armstrong Atlantic State University in Savannah, GA.
References
- Rose EAC. Strongyloides stercoralis.
http://www.emedicine.com/emerg/fulltopic/topic843.htm . Accessed
October 22, 2008. - Fardet L, Genereau T, Cabana J, et al. Severe Strongyloidasis in
Corticosteriods treated patients. Clin Microbiol Infec.
2006;12:945-947. - Vadlamudi RS, Chi DS, Krishnaswamy G. Intestional Strongyloidasis
and hyperinfection syndrome. Clin Mol Allergy. 2006;5:8. - Bianchi PG, Silva FSC, Barros MT, et al. A Rare Intestinal
Manifestation in a Patient with Common Variable Immunodeficiency and
Strongyloidasis. Int Arch Allergy Imm. 2006;140:199-204. - Currie BJ, McCarthy JS. Strongyloides stercoralis Infection as a
Manifestation of Immune Restoration Syndrome? Clin Infect Dis.
2005;40:635. - Karunajeewa H, Kelly H, Leslie D, et al. Parasite-Specific IgG
Response and Peripheral Blood Eosinophil Count Following Albendazole
Treatment for Presumed Chronic Strongyloidiasis. J Travel Med.
2006;13(2);84-91.