Within two miles of each other in western Pennsylvania are
the two largest hospitals among those served via the University of
Pittsburgh Medical Center (UPMC) system. UPMC Presbyterian, a 997-bed
academic hospital, is designated as a Level 1 Regional Trauma Center. UPMC
Shadyside is a 517-bed tertiary-care facility. In 2008, Presbyterian and
Shadyside clinical chemistry laboratories processed 4.5 million and 1.09
million billable tests, respectively. With test
volumes increasing yearly by 8% to 10%, implementing technologies to meet
their high-volume 24-hour testing demands was critical. In a concerted
effort to standardize laboratory testing and improve patient care, UPMC
sought total laboratory automation.
Choosing an automated system
The laboratory leadership employed a multidisciplinary
group to evaluate, select, and implement the “best fit” automation system.
The Laboratory Automation Group (the Group) included laboratorians (clinical
chemists, pathologists, and medical technologists), laboratory information
system (LIS) analysts, clinicians, nurses, and representatives from the
offices of UPMC's Facilities Management, as well as its Center for Quality
Improvement and Innovation.
The overall goal was to improve patient care through
accuracy and consistency in laboratory analyses. The strategic goals of the
UPMC Automated Testing Laboratories were to:
- install new chemistry and immunochemistry
analyzers, and an automated platform with sample management
capabilities; - integrate sample analyses and sample management;
- improve and standardize test turnaround times
(TAT), and improve the management of analyses through interactive
management software (middleware); - reduce multiple blood collections and aliquot
preparation; pre-analytical, analytical, and post-analytical errors; and
operation costs; - minimize laboratory staff exposure to biohazards
associated with sample processing; and - increase test-volume growth potential.
The Group's first overall task was to identify companies
with commercially available laboratory-automation systems capable of
satisfying the overall objective. The Laboratory Automation Group was then
separated into five teams. While supporting the Group's main objective —
improving patient care through accuracy and consistency in lab analyses —
each team delineated its specific needs. The Sample Management team sought
automation technologies requiring minimal operator intervention for sample
acquisition, sample integrity assessment, centrifugation, aliquoting,
storage, and sample retrieval.
The Instrument and Methods team wanted automation systems
that offered comprehensive chemistry and immunochemistry test menus with the
potential of test expansion. At the time of the project, the labs' analyzers
were supplied by a variety of companies; identifying one that offered both
general chemistry and immunochemistry analyzers was important. Cost of
operation, as well as system reliability and flexibility, were equally
significant.
The LIS team itemized the cost of potential software
upgrades needed to ensure the highest performance of the automation system.
The system's scalability and its ability to satisfy security and regulatory
compliance were also determined.
The Site Planning and Facilities Preparation team
assessed the facility infrastructure needed to support the new automation
system. This included evaluation of such factors as appropriate space
accommodation for both staff and instruments, wiring for normal and
emergency power, additional lighting, water access and drainage, and
appropriate ventilation.
The Transition to Automation System team evaluated the
companies' technical support, user training courses, and continuing
education sessions. This team identified areas in the laboratories' current
workflow processes that needed to be addressed prior to the implementation
of the new automation systems.
Automation improves workflow
After a thorough review of four commercially available
automated laboratory systems, the Group selected one that demonstrated cost
efficiency, user friendliness, reliability, and robustness. The total
laboratory automation system installed in March 2009 at Presbyterian
(February 2009 at Shadyside) is comprised of sorters, centrifuges,
aliquoters, general chemistry and immunochemistry analyzers, and a
refrigerated storage (stockyard) — all connected to a robotic track. Samples
are automatically managed once loaded into the inlet station by the
operator. The system scans the bar-coded sample, and the patient
demographics and test requests are downloaded.
Periodically, samples are scanned as they are transported
along the robotic track, allowing the operator to be cognizant of samples'
locations at all times. The system loads and unloads centrifuges, de-caps
samples, and detects sample volume. Samples requiring additional offline
testing are aliquoted from primary tubes to daughter tubes by the system.
Samples analyzed online are routed to the online chemistry and
immunochemistry analyzers.
Following analysis, samples are re-capped and transported
to the stockyard (stores up to 3,200 samples). In the event that a physician
requests additional testing from an earlier sample, the automated system
will retrieve the stockyard sample and transport it to the appropriate
analyzer. Samples analyzed offline (in-house or send-out testing) or those
requiring special attention are diverted to the outlet station for operator
intervention.
The automation system manages samples from beginning to
end. The middleware autoverifies results that pass pre-established criteria
and files results to patient records electronically; alerts the operator of
abnormal results that require further review; notifies the operator of the
sample's pre-analytical status; and prompts the operator of samples that
require offline dilution.
“The middleware technology enabled us to implement
autoverification for all chemistry and immunochemistry testing. With the
elimination of manually resulting normal results, the lab staff have more
time to manage critical results,” says Raymond Bezila, MLT, administrative
director for Automated Testing.
In addition to installing total laboratory automation,
the Group also took advantage of re-educating the hospital staff on sample
management. Prior to the automation install, the clinical chemistry labs
relabeled between 40% to 60% of samples received. Relabeling reduced lab
performance because it added a minute to TAT for results. Relabeling has the
potential to introduce identification errors and is not cost effective.
Moreover, the laboratory received a high volume of samples with accompanying
paper requisitions that required manual order entry. Paper requisitions
added three minutes per test order and increased TAT for results.
Another area of concern was tube sizes and types. Before
automation, the lab accepted samples in a variety of tube sizes and types.
It was necessary, however, to standardize the tube sizes since the new
system requires 13×100 vacutainer tubes. The UPMC Automated Line
Implementation Improvement Project was instrumental in developing a learning
module entitled “Correct Collecting, Labeling, and Sending samples to the
Lab: The Right Way Every Time.” The laboratory has seen significant
improvement in sample labeling. The installation of label printers on
hospital units has significantly reduced (by 21%) the volume of paper
requisitions received by the lab. To date, correct tube sizes have been
stocked on every unit, and tube sizes are now standardized between the two
hospitals; 89% of samples received can be placed directly onto the
laboratories' automated systems without operator intervention.
Laboratory optimization
Nine months have passed since the conclusion of the total
laboratory automation project, and performance has been enhanced
significantly. The automation system directly affects the laboratory staff,
the hospital community, and, most importantly, the patients.
“The automated line project represents state-of-the-art
in high-volume laboratory testing,” says Alan Wells, MD, DMSc, and vice
chairman of Pathology. “Laboratorians now have the technology that improves
workflow of routine testing and creates the capacity to focus closely on the
most difficult cases.”
The first six months of operation challenged the
operators as they increased their familiarity with the technology, while the
hospital community became compliant with revised sample-processing
procedures. Since both Presbyterian and Shadyside have identical automation
systems, each campus can perform its own testing; and the need for sharing
samples between the laboratories is reduced. Testing is now standardized
between the two because it is not unusual for patients to receive treatment
at both hospitals.
A meaningful reduction in the recorded volume of samples
that med techs manually handle translates to reduced exposure to biohazards
and reduced risk of injury. Med techs have more time to devote to
troubleshooting critical cases, performing quality-improvement research
studies (in collaboration with residents and researchers), and researching
and developing new assays. Currently, the laboratory staff is being
cross-trained, which not only presents development opportunities for
individuals but also creates depth and flexibility for laboratory
operations.
The hospital community has benefitted from the faster
STAT and routine turnaround times, which are surprisingly comparable and
suggest that in the future, distinguishing STAT from routine testing will be
unnecessary, because all samples will be analyzed STAT. The TATs for add-on
testing have improved and are directly related to the automation system's
ability to retrieve the sample with minimal human intervention. Med techs no
longer have to physically locate and retrieve the specific sample, which
once could take a half hour.
Prior to implementing the automation system, serum
indices were graded by visual inspection. The lab has begun the process of
standardizing the assessment of serum indices. Hemolysis, lipemia, and
icteria are detected via a spectrophotometry method, and semiquantitative
unitless values are provided for each sample on the chemistry analyzer.
Standardization of the pre-analytical, analytical, and post-analytical
phases has an enormous impact on ensuring the reliability of test results.
Patients have benefited with the consolidation of testing because the volume
of blood necessary to perform testing has decreased, reducing the need for
multiple blood draws, thus saving the patient time and discomfort.
The success of total laboratory automation at
Presbyterian and Shadyside is a direct result of teamwork among the vendor,
laboratory leadership and staff, and the hospital community. Implementation
of total laboratory automation requires change for the laboratory staff as
well as for the hospital community (nurses, phlebotomist, and clinicians).
Automation is not a substitute for laboratory personnel but a complementary
technology that aids in optimizing laboratory performance.
As performance through automation continues to be
optimized, the clinical chemistry laboratories at Presbyterian and
Shadyside now are truly living up to their names as UPMC Automated
Testing Laboratories.
Octavia M. Peck-Palmer, PhD, is assistant professor
in the Department of Pathology at the University of Pittsburgh, and
medical director at the UPMC Presbyterian and Shadyside Automated
Testing Laboratories in Pittsburgh. UPMC includes 15 general hospitals
in western Pennsylvania, related clinics and healthcare facilities, and
partnerships in five international hospitals.
Applying LEAN management to automation
By Scott Henwick, David Chow, and Sabrina Smith
Faced with shrinking budgets, growing volumes, and
personnel shortages, clinical laboratories are increasingly moving to
automation to maximize output and efficiency. But some are finding that
the anticipated rewards of automation are not necessarily automatic. In
fact, such moves may actually highlight a lab's hidden operational
inefficiencies, as getting samples to the automated system becomes the
critical link and the rate-limiting step. This, however, can present an
opportunity to implement LEAN practices in order to reap the full
benefits of automation.
This was the case with BC Biomedical Laboratories in
2006 when we increased the level of automation to address our rapidly
growing Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) testing volumes. BC Biomedical is the
largest physician-owned lab in British Columbia, Canada, serving 1.8
million patients and running 25,000 tests per day. At the time, our
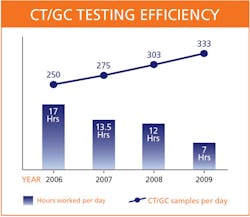
CT/GC testing volume was at 250 samples per day and rising by about 10%
per year.
We acquired a system which replaced manual pipetting
involved in sample transfers, amplification, and analysis steps with
robotics, with the objective of saving time as well as reducing manual,
repetitive tasks. We incorporated the new system into the current workflow
and quickly realized that our processes, which were previously adapted to
manual CT/GC processing, were not taking full advantage of the automation
the new system could deliver. We questioned if we could achieve higher
productivity by adjusting how we handle pre- and post-analytical steps, as
well as those that interact with the system. In order to make process
changes that would maximize the productivity and efficiency of the new
system, we turned to LEAN.
Specifically, we invited a LEAN/Six Sigma consultant to
perform a process review. He worked with our entire team of two clinical
supervisors, 30 medical technologists, and 15 lab assistants for three days
examining and applying LEAN principles to each step of our CT/GC sample
processing. What followed was a journey toward enhanced productivity and
cost efficiency, which inspired our staff to apply this continuous
improvement tool across all aspects of our work.
We worked with the consultant to make changes that
brought dramatic productivity improvements in just the first few days. This
gained the immediate support of our entire team — many of whom had been
hesitant to change — for the LEAN process. Over time, we implemented more
than 200 process improvements throughout the department, many of which were
small, incremental changes but all of which contributed to dramatic
productivity gains.
A new way of looking at workflow
Working with the consultant, the first thing we did was
to adopt the “single-piece flow” approach, a core tenet of LEAN, to sample
processing. Single-piece flow embraces the importance of considering each
sample individually, rather than as part of a batch in order to avoid
build-up of samples at any given stage, thus improving efficiency.
Specifically, decisions are made about each sample in real time as it moves
through the system, and problem samples are set aside for processing later
so they do not delay other specimens.
The single-piece flow concept also helped us think
differently about how we use the instrument. For example, we started
preparing the specimens when we had just one of two trays full (46 samples),
rather than waiting for a full batch of specimens on both trays (92
samples). This enabled us to reduce instrument lag time every morning and to
complete the runs earlier each afternoon. We also began processing samples
at the end of the day, so they would be ready the next morning. This
decreased instrument lag time in the morning from 90 to 30 minutes — which
freed up our techs for other tasks while the machine is running.
One area where single-piece flow helped eliminate waste
was in the central-processing area. This is where samples first arrive in a
large bin from doctors' offices and are labeled and entered into the LIS
system. Prior to LEAN implementation, we would enter all samples from that
box, label them all, and then return them to the bin unsorted. This approach
was inefficient since it created a lag in the system and was prone to
labeling errors. By mapping and optimizing the accessioning process, we
saved about 30 minutes in the first iteration of the new process. As we
further optimized the process by running pilot projects and refining the
physical workspace, the incremental time savings added up. Additionally,
this change to single-piece flow helped cut errors in central processing by
22%. By working with one specimen from beginning to completion, single-piece
flow eliminated the potential for mislabeling specimens.
Standardizing the work
Standardization of work processes is another fundamental
LEAN principle. Previously, each staff member had developed his own way of
performing various manual tasks. These included creative “work-arounds” for
typical process problems that might arise. When we installed a more
automated instrument and tried to apply to it our ad hoc processes, our work
system did not run smoothly, especially for processes that required a
handoff to another person. In contrast, by standardizing how we performed
each process, we were able to identify and institute best practices.
For example, previously, each technologist had his own
technique for loading samples into the instrument racks. The technologist
would typically label the sample before placing it in the rack and would
later go back to pick up each sample from its slot in order to scan the bar
code. To streamline the process, we established that all bar codes would be
scanned before being applied to the samples and that all samples would be
stacked from right to left, beginning in the back. These changes enabled us
to save 20 to 40 minutes per day. Additionally, by loading samples from
right to left, we were able to avoid having to move the wand cord out of the
way each time we loaded a sample. This allowed us to save a couple of
seconds per specimen, which by the end of the day could add up to several
minutes.
We also standardized work areas. For example, we used to
organize work lists into binders. For greater efficiency, we organized
paperwork into folders, each labeled by day of the week. We also color-coded
the racks to easily identify them. Additionally, we taped off and labeled
each work area. These changes enabled us to save several minutes each day.
Further, the physical separation of the specimen handling area from other
tasks such as administrative tasks minimized the risk of contamination.
Further, we reconfigured work spaces so that we all worked from left to
right, in a linear fashion. This increased efficiency and further minimized
risk of contamination.
Measurable benefits
In just two years, BC Biomedical's CT/GC volume increased
22% to more than 300 samples per day, while we maintained our current head
count of two technologists plus one-quarter of a lab assistant's time
dedicated to CT/GC testing. Our current staffing level for this bench can
handle an additional 25% capacity due to the continual process improvements
we have made in this area. Further, we are now able to complete the day's
CT/GC run by 2:30 p.m. vs. 5:30 p.m. previously. This means that doctors are
more likely to get results and, thus, may be able to treat patients a day
sooner.
Our adoption of LEAN has also produced less quantifiable
benefits, namely, reduced stress level of our staff, boosted staff morale,
and superior staff retention. All levels of our staff feel empowered because
each one has had a voice in our LEAN implementation. While our consultant
gave us the initial ideas for how to implement LEAN systems, our staff came
up with most of the process changes we implemented — and continue to
challenge ourselves to further improve our processes.
The move to LEAN has also changed how we communicate with
one another. We now have daily five-minute team meetings, usually first
thing in the morning, during which we identify and resolve work-process
issues we are experiencing. This enables us to fix problems quickly and
makes weekly staff meetings much more efficient. We also put in place a
formal workflow review process, which we conduct at least twice a year as a
group. Our LEAN efforts for CT/GC testing were so successful we were then
able to secure management approval to implement LEAN management throughout
the microbiology lab. We subsequently deployed it to the parasitology,
bacterial I.D., resistance testing, and blood-culture areas, all with
similar success.
Looking ahead
We plan to continue applying LEAN principles to work
processes, particularly as we further increase automation throughout the
lab. We have recently installed the next-level, fully automated system that
incorporates DNA extraction for the ever-growing CT/GC volumes. This time,
the move to increased automation is paying off immediately: In workflow
analysis, we are achieving completion of testing duties by 11:30 a.m. and
are utilizing about half of the FTE component compared to the current
methodology.
LEAN has given us tools to better assess the actual
workload impact of bringing any new technology into the laboratory.
Additionally, with LEAN we are now more confident that before we go “live”
with production, we have mapped out the process to a more efficient starting
state. And by standardizing work processes, we have created a common
baseline upon which to run pilot projects to continuously improve the
process.
Our success with LEAN in the microbiology department has
also prompted its adoption across the entire organization. The lessons
learned in microbiology about single-piece flow, eliminating waste, and
standardization of work processes are now being applied successfully to all
areas of the organization.
Scott Henwick, MD, F(RCPC), clinical director of microbiology,
David Chow, manager of microbiology and parasitology; and
Sabrina Smith, supervisor of microbiology — all
employed at BC Biomedical Laboratories Ltd. in Surrey, British Columbia,
Canada — used a BD Diagnostics' LEAN/Six Sigma consultant, and first
installed a BD Viper System, which was followed by the installation of the
BD Viper System with XTR technology.