CONTINUING EDUCATION
To earn CEUs, see current test at
www.mlo-online.com under the CE Tests tab.
LEARNING OBJECTIVES
Upon completion of this article, the
reader will be able to:
- Discuss the progress of transfusion medicine since 1900.
- List the three most common causes of transfusion-related deaths.
- Discuss two advances in decreasing adverse events from blood
transfusion. - Discuss two important findings that have changed blood-component
transfusion practices. - Discuss a current blood substitute in terms of availability.
- List one future application of cell therapy.
Although
animal-to-human blood transfusions had been performed for several
thousand years, and human-to-human transfusions were attempted in the
14th and 18th centuries, it was not until 1818 that a British
obstetrician, James Blundell, reported the first successful
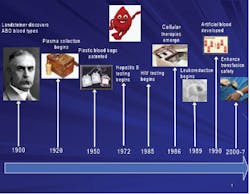
human-to-human transfusion.1 In 1900, with the discovery of
the ABO blood group by Nobel Prize winner Karl Landsteiner,2
the field of “transfusion medicine” was to evolve into today’s
multifaceted science. The practice of transfusion medicine now
encompasses blood collection, testing, manufacturing, storage, and
distribution; traditional blood transfusion and advanced cellular
therapies; recombinant technology, blood substitutes, and engineered
tissues; improved transfusion safety; clinical medical practice
specialty; and regulatory oversight (see Figure 1).
Landmark research findings have preceded the
practice of blood transfusion today; and more research is needed in
order to provide the best, safest, and most cost-effective blood and
cellular therapies to patients requiring these treatments. How are we
doing today? What innovations lie ahead?
Blood collection
The most recent U.S. National Blood Collection
and Utilization Report (NBCUS 2007) indicates that the number of
allogeneic whole blood/red blood cell (WB/RBC) collections (16,174.000)
before testing increased 5.4% from 2004 to 2006.3 The report
also states that allogeneic RBC transfusions increased 3.3% in 2006 to
14,650,000. Apheresis RBC collections increased 96.4% in 2006 due to the
U.S. Food and Drug Administration (FDA) approval and introduction of
two-unit RBC collections by automated equipment. This technology will
allow blood centers to provide an increased RBC inventory to hospitals
and help prevent RBC shortages that have plagued RBC availability during
the summer and holidays.
Platelet-unit availability is always a concern
due to the five-day expiratory date. The report indicates that the
number of available platelets in 2006 was 10,388,000, and the number of
platelet transfusions was 9,092,000. Although the increase in collected
and transfused platelets was not statistically significant, these data
indicate that demand will outpace supply unless more platelet donations
can be obtained (apheresis collections becoming the primary method of
donation). The demand for plateletpheresis is due to increased
bone-marrow transplantation procedures requiring platelet support,
increased trauma cases, and an increase in hematological fragile
surgical/medical/oncology patients. An increase in the current 5% of
eligible blood donors who routinely donate blood in this country is
emergently needed.
Blood safety
Transfusion of blood
components is safer today than at any time. Advances in testing technology
with the use of nucleic-acid testing,
or NAT, and the addition of West Nile virus and Chagas disease tests
performed on donated blood has provided additional layers of safety from
acquiring transfusion-transmitted diseases. The estimated risk of
transfusion-transmitted diseases per unit of blood component transfused is
shown in Table 1.
Table 1. Risk per unit transfused of selected transfusion-transmitted
diseases.4
Febrile non-hemolytic transfusion reactions have
steadily declined since the advent of leukoreduction (removal of white
blood cells by filtration or automation). Leukoreduction also minimizes
alloimmunization and immunomodulation events from blood- component
transfusion. With the United States approaching 100% leukoreduction,
decline in these adverse events will be realized.
Transfusion reactions have also seen a decline in
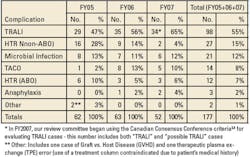
several categories but an increase in others. Total
transfusion-recipient deaths reported by the FDA declined in the FY 2007
(see Table 2). Decline was noted in ABO incompatibility but increased
for transfusion-related acute lung injury (TRALI). This increase, most
likely, relates to improved scientific knowledge and education about the
signs/symptoms of this entity.
Table 2. FDA transfusion fatalities for FY05-07.5
Also noted in this report is the decline of
reported transfusion fatalities associated with platelets collected by
apheresis. Bacterial contamination of blood collections has seen peaks
and troughs since collection of plasma started during World War I (WWI)
and continued in massive volumes in WWII with the use of glass bottles
(see Figure 2). Glass bottles, besides being breakable, required an air
vent for flow, which made these containers an “open system,”
facilitating the entrance of bacteria into the container. With the
introduction of plastic blood containers in the late 1950s, a closed
system was maintained for whole-blood units. The use of plastic blood
containers, however, allowed for the introduction of blood “component”
manufacturing and whole-blood-derived platelets stored at room
temperature then became a source of increased bacterial contamination.
Many of these contaminants were from the skin flora, and several safety
measures were recently introduced to decrease platelet bacterial
contamination.
In May 2004, the AABB (American Association of
Blood Banks) required all AABB-accredited facilities to “have methods to
limit and detect bacterial contamination in all platelet components.”7
Collection facilities and hospital-transfusion services began using
several manual techniques (pH, glucose level, platelet swirling) to
detect possible bacterial growth. Increased use of culture-based methods
introduced by many facilities for both whole-blood-derived components
and apheresis platelets is also noted. The FDA also licensed several
automated devices to allow for “quality control” (QC) of platelet
components — the platelet-collection process does not demonstrate a
higher contamination rate that the predefined rate for the device.8
The FDA, as of this writing, has not licensed any of these devices as a
“release test” — the platelet component being released/issued does
not contain bacteria.
Another advance in eliminating bacterial
contamination of whole-blood-derived components is the use of a
blood-diversion pouch (see Figure 3). During whole-blood collection the
first several milliliters of blood which contains a probable
bacteria-laden skin-plug from the venipucture is diverted into a small
blood container prior to the filling of the main collection bag. This
collection process has resulted in up to 50% reduction of contamination
of whole-blood components.9
Figure 3. Blood-collection diversion pouch used to decrease bacterial
contamination and allow collection of donor testing samples.
A major risk to transfusion safety is mislabeled
specimens, wrong blood in collection tubes (WBIT), and transfusion to
the wrong patient. These errors in pre-analytical and post-analytical
hospital procedures have not abated during the entire history of
laboratory medicine. The Joint Commission issued Patient Safety
Guidelines in 2005, trying to spearhead hospitals to decrease
patient-specimen mislabels and WBIT by requiring the use of two patient
identifiers (not a room number) Transfusion services have required
patient-identity verification by the transfusionist and a witness for
many years. Mistransfusions continue today, however, and alternate
processes are being implemented to ensure a safe blood transfusion.
Bar coding is becoming a standard technology in
laboratory practice for sample identification from bedside to analytical
testing. Hospitals have been slow to implement bar coding on patient
wristbands due in most cases to the cost of such technology. Bar-code
technology (machine-readable) includes a variety of devices —
one-dimensional and two-dimensional bar codes, as well as radio
frequency identification, or RFID, tags. This technology is also
expanding to other areas of hospital patient care requiring proper
patient identification (medication given via “smart” infusion pumps).10
When a can of beans purchased in most grocery stores is more accurately,
consistently, and correctly identified than a blood transfusion
recipient, has not the time arrived for investing in this new technology
to prevent possible deaths related to a mistransfusion?
Blood-transfusion practices
Over
the past 20 years, researchers have investigated the appropriate indicators
for blood-component transfusion. With the blood supply being both volatile
in availability and not at “zero-risk,” change in patient-transfusion
practices is providing optimal patient care with lower risk. Several seminal
publications have demonstrated that physicians “over transfuse” blood
components and may even increase the morbidity and mortality rates of
transfusion recipients.
A multicenter randomized control trial (RCT) of
transfusion thresholds in critically ill patients in intensive-care
units demonstrated that a liberal RBC-transfusion protocol (maintaining
the hemoglobin level between 10 g/dL and 12 g/dL) resulted in a 5%
higher mortality rate when compared to a restrictive RBC protocol
(maintaining hemoglobin levels between 7 g/dL and 9 g/dL).11
Although such a restrictive hemoglobin “transfusion trigger” may not be
appropriate for all patients, less is better.
An RCT evaluating the optimal threshold for
allogeneic platelet transfusions for prophylactic treatment of patients
with acute myelogenous leukemia demonstrated that a lower platelet
“transfusion trigger” (10,000/uL) resulted in no more bleeding episodes
than those patients transfused at higher levels (20,000/uL).12
Again, in this study population, less is better.
Fresh frozen plasma components (FFP thawed,
thawed plasma, cryoprecipitate-reduced plasma, cryoprecipitate)
transfusions have also been over utilized. Although a 1.9% decrease in
FFP plasma components was noted in 2006 from 2004 transfusions,3
inappropriate FFP transfusions continue. The most common reason to
prophylactically transfuse FFP is because of a minimally elevated
prothrombin time (PT) and international normalized ratio (INR).13,14
Due to the physiologic exponential rate curve between percent of factor
concentration vs. mildly elevated PT/INR, larger volumes of FFP will not
significantly improve the PT/INR values. Also, mildly elevated PT/INR
( predict bleeding, including procedures such as minimally invasive liver
biopsy or catheter insertions. The risk-benefit ratio is high for these
blood components (i.e., TRALI), so less is better.
The new “kid on the block” in transfusion
medicine is cellular therapy. Hematopoietic progenitor cell
collection/HPC (peripheral and cord blood) and transfusion is the newest
modality in bone-marrow transplantation (BMT). A 25% increase in
apheresis HPC collections and 208% increase in cord-blood collections
were noted between 2004 and 2006.3 Although a few patient
diseases require the traditional bone-marrow-collected HPC, most BMT
programs are utilizing apheresis and cord HPC collections/transfusions
as their primary transplant support. In this area, more is
better.
Future-transfusion-medicine innovations
What future technology awaits the
transfusion-medicine community? Progress is being made in two important
areas of routine blood transfusion: 1) pathogen inactivation and 2)
blood substitutes. Pathogen inactivation (PI) techniques are varied
depending on the component under investigation (cellular, plasma). PI
has the potential to eradicate all currently known
transfusion-transmitted viruses and bacteria, and even those not yet
discovered.15 The success of such technology would also
eliminate the need for bacterial detection, infectious-disease marker
testing, and cellular- component irradiation. On the negative side,
however, are the toxicities, neoantigenicities, efficacy of PI component
transfusion in certain recipient populations (premature infants,
neonates, pregnant patients), and cost. Although this is an exciting
time in PI, caution is required based on the risk/benefit to society.
Blood substitutes have been slower to reach
inclusion in the armamentarium of transfusion medicine. Only one product
(PolyHeme, Northfield Laboratories, Evanston, IL), a human
hemoglobin-based polymerized oxygen carrier (HBOC), is nearing
submission to the FDA for a biologic license application.16
As with most of the HBOC products, this product is to be used for
temporary oxygen delivery until the patient is stabilized and
routine RBCs are available for transfusion. With a shelf-life of 12
months and 50 g of modified hemoglobin, the potential for use in
critical-care emergencies holds promise. Many of the HBOC trials have
been discontinued due to untoward organ toxicities.
Future developments in tissue engineering (ex
vivo
expansion), hopefully, will allow for use of various cells lines (e.g.,
liver, neuronal, cardiac valves, muscle, blood) to be available to
specifically replace non-functioning tissue and treat diseases that
consist now only of supportive-treatment modalities. New
cellular-component storage mediums are also being evaluated to prolong
the shelf life of stored donor blood and provide enhanced transfusion
efficacy for the recipient. Will the blood donor become non-existent?
Not in our grandchildrens’ lifetimes at the earliest. But that day
will come. Stay tuned.
William B. Lockwood, PhD, MD, is a clinical professor in the Department of
Pathology and Laboratory Medicine at the University of Louisville in Kentucky. He also is director of Transfusion Services and Tissue/Bone Bank at the University of Louisville Hospital; and director, Transfusion Services, Tissue/Bone Bank and Coagulation Laboratory at the Norton Hospital-Kosair Children’s Hospital, also in Louisville.
References
- Greenwalt TJ. The short history of transfusion medicine.
Transfusion. 1997;37:550-563. - Lansteiner K. Zur Kenntnis der antifermentativen, lytischen and
agglutinierenden Wirkungen des Blutserums und der Lymph. Zbl Balk.
1900;27:367. - U.S. Department of Health and Human Services. The 2007
Nationwide Blood Collection and Utilization Survey Report.
Washington, DC: DHHS, 2008. - Bihl F, Castelli D, Marincola F, Dodd RY, Brander C.
Transfusion-transmitted infections. J Transl Med. 2007;5:25
Available at
http://www.translational-medicine.com/contents/5/1/25 . Accessed
on December 8, 2008. - Fatalities Reported to FDA Following Blood Collection and
Transfusion. Annual Summary for year 2007. Center for Biologics
Evaluation and Research, Bethesda, MD. Available at
http://www.fda.gov/cber/blood/fatal07.htm . Accessed on December
8, 2008. - Blood transfusion set, ca 1900. Oregon Health Sciences
Historical Collections & Archives. Available at
http://content.ohsu.edu . Accessed on December 8, 2008. - Price TH, ed. Standards for Blood Banks and Transfusion Services
(ed 25). Bethesda, MD: AABB; 2008; p. 11. - Kaufman RM. Platelets: testing, dosing and the storage
lesion-recent advances. Hematology. (Am Soc Hematol Educ
Program). 2006:492-496. - McDonald CP, Roy A, Mahajan P, et al. Relative values of the
intervention of diversion and improved donor-arm disinfection to
reduce the bacterial risk from blood transfusions.
Vox Sang. 2004;86(3):178-182. - Dzik WH. Technology for enhanced transfusion safety.
Hematology. (Am Soc Hematol Educ Program). 2005;476-482. - Hebert PC, Wells G, Blajchman MA: A multicenter randomized
controlled trial of transfusion requirements in critical care.
NEJM. 1999;340:409-417. - Rebulla P, Finazzi G, Marangoni F, et al. The threshold for
prophylactic platelet transfusions in adults with acute myeloid
leukemia. NEJM. 1997;337:1870-1875. - Triulzi DJ. The art of plasma transfusion therapy. [editorial].
Transfusion. 2006;46:1268-1270. - Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma
transfusion on prothrombin time and bleeding in patients with mild
coagulation abnormalities. Transfusion. 2006;46:1279-85. - Webert KE, Cserti CM, Hannon J, et al. Proceedings of a
consensus conference: pathogen inactivation-making decisions about
new technologies. Transfus Med Rev. 2008;28:1-34. - PolyHeme® Multicenter Phase III Trial. Available at
http://www.northfieldlabs.com/polyheme.html . Accessed December
8, 2008.
MLO’s
Continuing Education Test is available online only.
Print out and mail a copy with your check, or use the new online CE test
and convenient online payment feature available through the auspices of
Northern Illinois University.
Go to www.mlo-online.com and
look under CE Tests.
Plus
Nitric-oxide bioactivity depletion:
An added storage lesion in banked blood
By Faon Rodriguez, MS, and Diana Ramirez, MS
There is more to a
blood transfusion than to increase the oxygen-carrying capacity of red-blood
cells (RBCs). Blood transfusions should be able to enhance vasodilation,
open blood vessels, and increase blood flow to hypoxic tissue. Blood
transfusions should also help improve RBC rheology to facilitate flexible
transport of RBCs through tiny capillaries. If, however, blood vessels
vasoconstrict or if the RBCs become rigid, then the blood transfusion may be
less effective.
An effective blood transfusion is one that not only
raises the hematocrit to the normal range but also enhances arterial
relaxation and vasodilation. In man, the enzyme that produces nitric oxide
(NO) from L-arginine is called nitric-oxide synthase The vasodilator gas,
nitric oxide, is carried by hemoglobin in the form of S-nitrosothiol (SNO)
(see Figure 1 online). Nitric oxide released from S-nitrosothiol helps relax
smooth muscle surrounding the tubules of arteries, arterioles, and
metarterioles (see Figure 2 online). When smooth muscle relaxes, the
arterial blood vessels expand, leading to vasodilation. Thus, wide-open
vessels improve the blood flow from arteries to capillaries, making the
transport of oxygen to tissue more efficient. Ultimately, in the
mitochondria, oxygen contributes to produce adenosine triphosphate (ATP)
from adenosine diphosphate (ADP) by electron-transport and oxidative
phosphorylation. ATP is used as an energy source by tissue. Blood
transfusions should be characterized by how well RBCs facilitate
nitric-oxide bioactivity to open blood vessels, rather than by how much
oxygen they can deliver to tissue.
Recently, Jonathan S. Stamler, MD, et al, and Timothy
J. McMahon, MD, PhD, et al, at Duke University Center (DUMC), have shown how
nitric-oxide bioactivity helps RBCs ferry oxygen to tissues by opening tiny
vessels during hypoxic vasodilation.1,2 They also report that
when RBCs leave the body during blood-banking donations, nitric-oxide
bioactivity and S-nitrosohemoglobin in RBCs begin breaking down almost
immediately. This type of storage lesion continues during the shelf life of
banked blood. The depletion of nitric-oxide bioactivity, along with the
decrease in concentration of 2,3-diphosphoglycerate, or 2,3-DPG, both affect
vasodilation, compromising oxygen delivery to tissues. Thus, it does not
matter how much oxygen hemoglobin carries; if the blood vessels do not relax
and open, oxygen cannot be delivered effectively to tissue. Therefore, the
unimpeded flow of blood throughout the blood-vessel bed is vital for the
efficient transport of oxygen.
The primary function of the cardiovascular system is
to supply oxygen to tissues and organs in the body RBC transfusions are
commonly used to treat anemia, including euvolemic patients with congestive
heart failure, to increase oxygen delivery to hypoxic tissue. The RBC
functions as an O2 sensor contributing to the regulation of blood
flow and oxygen delivery, by releasing nitric oxide, depending on the
oxygenation state of hemoglobin. RBCs depleted of nitric-oxide bioactivity
do not always improve oxygen delivery during blood transfusions. There are
recent concerns about the benefit of blood transfusions to critically ill
patients due to immunomodulation and storage lesions found in banked blood.3
Blood transfusions can lead to vasoconstriction, congesting the flow of
oxygen in narrow passageways in the cardiovascular system — damaging the
very heart tissue that the blood was transfused to help.
Blood transfusions that lack nitric-oxide bioactivity
may become associated with ischemia, a dangerous drop in blood flow.
Investigators are suggesting that re-nitrosylation — adding a purified
aqueous solution of nitric-oxide gas into banked blood before transfusions —
can raise the concentration of S-nitrosohemoglobin in vitro which, in
turn, can amplify vasodilation in vivo. In fact, Dr. Stamler and the
anesthesiology and biochemistry teams at DUMC have demonstrated that canine
coronary-blood flow was greater during the infusion of rejuvenated re-nitrosylated
RBCs than during infusion of S-nitrosothiol-depleted RBCs. This article
reviews the literature on depletion of nitric-oxide bioactivity in banked
blood affecting vasodilation, blood flow, and oxygen transport to tissue,
and elaborates on current research about the rejuvenation of donated banked
blood by adding nitric oxide prior to transfusion.
Surprises at the cellular level happen all the time.
Nitric oxide or nitrogen monoxide is a formidable toxin commonly found in
nature as a gas and as a component of air pollution. For instance, it is a
pollutant produced by automobile exhaust fumes and by power plants. Oxygen
from the air and nitrogen combine at combustion temperatures or in the
presence of electrical energy to form nitric oxide. Nitric oxide is produced
by many cells in the body; however, its production by the vascular
endothelium — the innermost cell layer of blood vessels — is particularly
important in the regulation of blood flow. Today, we know that nitric oxide
plays a function in the cardiovascular system, the immune system, and in the
central and peripheral nervous systems. Furthermore, this simple gas, nitric
oxide, affects a variety of complex biological processes, including
blood-pressure homeostasis, platelet aggregation, and transmission of
signals by the nervous system.4 NO also plays a key role in the
activation of macrophages and cellular defenses against microbial pathogens.
It is a major pathophysiological mediator of inflammation and host-defense
mechanisms.
In the 1800s, Alfred Nobel invented dynamite, in
which one of the main components is the explosion-prone nitroglycerine.
Centuries later, nitroglycerine is being used as a vasodilator by patients
with chest pain, suffering with angina. Nitroglycerine is converted into
nitric oxide in the bloodstream, relaxing the muscle lining of vessels to
allow better blood flow.5 In the mid-1980s, scientists were
surprised to find out how nitric oxide was being produced meticulously in
human cells. Nitric oxide went from being an extraneous toxic and corrosive
gas to becoming an ubiquitous elixir of life. In 1987, Salvador E. Moncada,
MD, PhD, discovered the vital role of nitric oxide as a messenger in the
relaxation of muscle.6
In 1991, a team headed by K. E. Andersson of Lund University Hospital in
Sweden showed how nitric oxide was the principal neurotransmitter mediating
erectile function.7 In 1992, Science published a cover
story naming nitric oxide the molecule or the year. In 1998, Robert F.
Furchgott, MD, from the State University of New York; Louis J. Ignarro, MD,
from the University of California; and Ferid Murad, MD, PhD, from the
University of Texas, were given the Nobel Prize in Physiology for their
discoveries of nitric oxide as a signaling molecule in the cardiovascular
system.8
Non-biological functions of NO
Nitric
oxide should not be confused with a) nitrous oxide (HN2O), a general
anesthetic; or b) nitrogen dioxide (NO2), which is another poisonous air
pollutant; or c) nitric acid (HNO3). Nitric oxide is a very unstable free
radical turning, within seconds, into univalent radicals of nitrate (NO3)
in vivo and into nitrite (NO2) in vitro. Nitric oxide reacts with
ozone in the air to form nitrogen dioxide (2NO + O2 ? 2N02).
The synthesis of NO from molecular nitrogen and
oxygen ( N2 + O2 ? 2NO) requires elevated temperatures of greater than
1,000^0C. Internal-combustion engines have increased the concentration of NO
in the environment by automobile-exhaust fumes. The purpose of catalytic
converters is to minimize nitric-oxide emissions by catalytic conversion to
O2 and N2. Nitric oxide in the air can convert into nitric acid, which has
been implicated in acid rain.
Biological functions of nitric oxide
Nitric oxide is a lipophilic radical that readily moves across permeable
cell membranes via passive diffusion. Nitric oxide is one of the few
gaseous particles with biological-signaling capabilities. NO is known as
the endothelium-derived relaxing factor, or EDRF, and a liable free
radical with a half-life of about three to five seconds. It is
biosynthesized from L-arginine and oxygen to citrulline by several
nitric-oxide synthases, or NOS, enzymes and by the reduction of
inorganic nitrate. NO is known to be produced in bacteria but found to
act differently in mammals as a signaling molecule. Produced by many
types of cells including nerve cells and the endothelium, nitric oxide
is regulated by biofeedback and by the ability of superoxide anion and
superoxide dismutase to inactivate NO. Nitric oxide is also controlled
by the “on-and-off redox switch” — the reduction/oxidation potential
states of biochemical reactions.
Discussion
Banked-blood packed RBCs have had
most of their leukocytes and plasma removed. Packed RBCs undergo rigorous
testing before their use. The blood-banking industry does an extraordinary
job of manufacturing a safe and effective product. In order to prevent
transfusion-transmitted diseases, the Food and Drug Administration (FDA)
mandates testing for viral markers including hepatitis B; human
immunodeficiency virus 1,2, or HIV 1,2; human T-lymphocytotrophic virus 1,2,
or HTLV-1,2; cytomegalovirus, or CMV; serologic test for syphilis,
nucleic-acid testing, or NAT, for West Nile virus, and hepatitis C virus.9
In addition, in order to conserve red-cell survival and function, RBC
units are treated with additive solutions containing sodium chloride,
dextrose, adenine, monosodium phosphate, mannitol, sodium citrate, and
citric acid. RBCs also contain anticoagulants like citrate-phosphate
dextrose, or CPD; citrate-phosphate dextrose-dextrose, or CPD2D; or
citric-phosphate dextrose-adenine, or CPDA-1. The 42-day expiration date of
banked blood stored at 1^0C to 6^0C depends mainly on the type of additive
solutions used including AdsolR (Fernwall, Lake Zurich, IL), NutricelR (Pall
Life Sciences, Ann Arbor, MI), or OptisolR (Terumo, Somerset, NJ).10
RBCs collected using the Trima Accel Collection System (CaridianBCT,
Lakewood, DO) also have a shelf life of 42 days.
Approximately 13.9 million units of blood are
transfused to 4.8 million patients each year in the United States, and the
basis for approved use is determined by meeting regulations during
collection, processing, and storage.11 Banked blood is a
biological product that is under the scrutiny of many regulatory agencies
and the scientific community. The AABB (American Association of Blood Banks)
also publishes guidelines for a safe transfusion. Blood has both benefits
and risks, and, therefore, should be evaluated in the same manner as
medications. To make a better product, however, the industry has pursued the
idea of introducing synthetic banked blood, but its success remains to be
proven. Until then, investigators are proposing ways to improve the product
that is already at hand today. The current interest in the literature is
about nitric-oxide bioactivity found in the form of S-nitrosothiol, which is
crucial for the delivery of oxygen to tissues. Nitric oxide is not only
needed for RBCs to transport oxygen but also may be responsible for the
flexibility of the RBCs. When nitric-oxide levels decrease, the RBCs become
stiffer, making it more difficult for them to adapt their shape in order to
travel through the tiny capillary spaces during the delivery of oxygen (see
Figure 3 online).
Storage lesions include RBC rheology, the loss of
shape, and flexibility,12 the decrease in the concentration of
molecular modulators of oxygen binding (e.g., 2,3-DPG), the decrease in
nitric-oxide bioactivity, and the increase in RBC adhesiveness during
prolonged storage. Storage lesions in banked blood have been found to be
responsible for adverse outcomes, like those leading to increased mortality
rates after blood transfusion. Alterations in RBC rheology and adhesion may
exacerbate rather than correct ongoing ischemia and — at least, partly —
account, for the adverse effects of blood transfusions.
At the Cleveland Clinic Foundation, Colleen G. Koch,
MD, et al, examined data from 1998 to 2006 for patients who received RBC
transfusions. A total of 2,872 patients received 8,802 units of blood that
had been stored for 14 days or less (“fresh blood”); 3,130 patients received
10,782 units of blood that had been stored for over 14 days (“aged blood”).
After cardiac-surgery patients who were transfused, “aged blood” had an
increased risk of postoperative complications and reduced chance for
survival.13
Nobel laureate Dr. Ignarro, in his book No More
Heart Disease, indicates that the endothelial cells can get sabotaged by
a variety of health conditions that compromise the production of nitric
oxide. Some of the health conditions that add more stress to the blood
vessels and that inflict endothelium-cell damage include high blood
pressure; atherosclerosis; high blood-cholesterol levels; elevated blood
glucose; high low-density lipoprotein, or LDL; and cigarette smoking. The
endothelial cells produce nitric oxide to protect us from many diseases by
regulating blood pressure and blood flow. The endothelial cells, however,
have a much harder job producing nitric oxide in patients with hypertension,
coronary heart disease, or stroke.14 Thus, patients with
underlying cardiovascular disease who are in need of blood transfusions have
a much bigger challenge to process nitric-oxide-depleted banked blood,
especially if it is more than 14 days old.
Furthermore, patients with sickle-cell anemia have
abnormal hemoglobin, which is needed to deliver oxygen and nitric oxide to
tissue. Hemoglobin-S has a lower affinity for oxygen and, once deoxygenated,
the RBCs become distorted or sickled. Hemoglobin-S does not transfer nitric
oxide from heme to thiol as well as normal hemoglobin during S-nitrosohemoglobin
conformation The symptoms of sickle-cell disease are attributed to the
physical obstruction of blood vessels by distorted or sickled and rigid
RBCs.15 Thus, sickle cells become fragile, demonstrate
vasooclusion, and lead to hemolytic anemia. Consequently, sickle cells have
added disadvantages pertaining to blood-vessel dilation when tissue
experiences oxygen deficiency during hypoxemia. Sickle-cell patients who
receive blood transfusions may have the transfused RBCs accumulate in their
vascular system, impeding the free flow of blood and transportation of
oxygen to tissue. Relieving the vasoconstriction and restoring nitric oxide
to RBC membranes may help prevent the painful symptoms of sickle-cell
disease. Thus, there is an opportunity for clinical trials on the
therapeutic use of nitric oxide with sickle-cell patients.
There is growing interest to improve the safety of
our blood supply. Investigators at DUMC have shown that the level of S-nitrosohemoglobin
was reduced by 85% to 95% at storage days seven and 43 compared to day one.
They also noted a deficiency in vasodilotary activity in banked blood when
compared with “fresh blood.” Banked blood used for transfusion still has
some shortcomings, but researchers are now contemplating reducing some of
the storage lesions by replenishing nitrosylation in banked blood before
using it for transfusion.
Replenishing bioactivity modulators in banked blood
is nothing new. During the Vietnam era, United States Navy Physician C.
Robert Valeri and N. M. Hirsch showed that ”spiking” stored RBCs with
diphosphate glycerate and ATP precursors led to significant improvements in
cardiovascular function.16 Some establishments use rejuvenating
solutions, like RejuvesolR (Cytosol Labs, Lenoir, NC) which contains
pyruvate, inosine, phosphate, and adenine, to restore oxygen transport and
improve post-transfusion survival of RBCs.
It is being suggested that adding nitric oxide to
banked blood before its use could, theoretically, improve hemoglobin
nitrosylation and the ability of nitric oxide in S-nitrosothiols to dilate
and open blood vessels and, thus, prevent heart attacks and even death.
Investigators have demonstrated that replenishing banked blood with nitric-
oxide gas raises S-nitrosohemoglobin, or SNO-Hb, concentrations and restores
the hypoxic vasodilatory activity of RBCs.
The RBC is more than a “passive bag” full of
hemoglobin that transports oxygen. In fact, the RBC is a regulator of its
own destination. The matching of oxygen supply with demand requires
nitric-oxide bioactivity to increase blood flow in response to decreased
levels of oxygen in tissue. Increasing the hematocrit into the normal range
after a blood transfusion should be supplemented by increasing vasodilation,
blood flow, and oxygen transport. This can be achieved by adding nitric
oxide to banked blood prior to blood transfusions. Therefore, we support the
new paradigm from Joseph Bonaventura, PhD, for testing nitric-oxide
bioactivity in banked blood, once re-nitrosylation of banked blood becomes a
reality.17
There exist opportunities to further investigate nitric-oxide bioactivity as
suggested by Bonaventura which include testing of arterial and venous RBC S-nitrosohemoglobin
as a diagnostic indicator for transfusion; assaying hemoglobin re-nitrosylation
treatment of stored RBCs; verification of normalized RBC rheology before
transfusions; and verification of normalized RBC vasoactivity prior to
transfusion.
Allogenic, autologous, and directed blood
transfusions are not scrutinized with a risk/benefit analysis common for all
biologics. Furthermore, there are no regulations or clinical standards aimed
at examining the clinical outcome of an effective blood transfusion in
patients with respect to nitric-oxide bioactivity in vasodilation, blood
flow, and oxygen transport to tissue. Consequently, an opportunity exists
for clinical trials to evaluate the outcome and effects of transfusing
improved re-nitrosylated banked-blood products to patients. Thus, further
research is needed to measure the effectiveness of transfusions by testing
the concentration of nitric oxide in the form of S-nitrosothiols or S-nitrosohemoglobin
in the peripheral blood of patients who receive blood transfusions.
Adding soluble portions of nitric oxide to banked
blood is in its infancy, but this seems to be more promising than the
current results and developments seen with the manufacturing of a synthetic
blood product. The addition of nitric oxide to banked blood eventually may
need to undergo rigorous clinical trials, FDA approval, and re-evaluation of
the current 42-day expiration date. Nonetheless, the growing concern of
transfusing banked blood that is over 14 days old in patients undergoing
cardiac surgeries may help expedite more research and diligent acceptance by
the medical community and regulatory agencies. Re-nitrosylation of banked
blood with nitric oxide is the most promising project undertaken by DUMC
investigators to preserve more of our blood supply.
Faon Rodriguez, MS, is section supervisor at Florida Hospital, Celebration Health, FL, and
Diana Ramirez, MS, is transfusion service supervisor at Osceola Regional Medical Center, Kissimmee, FL.
Acknowledgements:
The authors want to thank these colleagues for their advice and comments
after reading this manuscript: Patrick J. O’Sullivan, laboratory
director, Florida Hospital, Orlando, FL; Pamela Hargrave-Thomas,
laboratory director, Osceola Regional Medical Center, Kissimmee, FL;
Theresa Palmer, assistant director, Florida Hospital, Kissimmee, FL; and
Kathryn Pearson, assistant director;
Gail S. Borysko, laboratory supervisor; and
Sonaly Cosme, medical technologist — all from Florida Hospital,
Celebration Health, FL.
References
- Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-Nitrosohemoglobin
deficiency: A mechanism for loss of physiological activity in banked
blood.In: Proceedings of the National Academy of Science (PNAS).
2007;104(43):17058-17062. - Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid
TS, Mulherin MA, Zhu H, Buck RD, Califf RM, and McMahon TJ. Evolution of
adverse changes in stored RBCs. In: Proceedings of the National Academy
of Science (PNAS). 2007;104(43):17063-17068. - Tinmouth A, Fergusson D, Ye EC, Hebert PC. Clinical consequences of
red cell storage in the critically ill. Transfusion.
2006;46:2014-2027. - Durner JR, Gow AJ, Stamler JS, Glazebrook J. Ancient origin of
nitric oxide signaling in biological systems. In: Proceedings of the
National Academy of Science (PNAS). 1999;96(25):14206-14207. - Chen LY, Mehta JL. Downregulation of nitric oxide synthase activity
in human platelets by nitroglycerin and authentic nitric oxide. J
Invest Med. 1997;45(2):69-74. - Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for
the biological activity of endothelium-derived relaxing factor.
Nature. 1987;327:524-526. - Stief CG, Holmquist F, Allhoff EP, Andersson KE, Jonas U.
Preliminary report on the effect of the nitric oxide donor SIN-1 on
human cavernous tissue in vivo. World Journal of Urology.
1991;9(4);237-239. - Furchgott RF, Ignarro LJ, Murad F. Nitric oxide as a signaling
molecule in the cardiovascular system. The Nobel Prize in Physiology
or Medicine. 1998; from website accessed November 11, 2007
http://nobelprize.org/nobel_prizes/medicine/laureates/1998/ . - Keeping Blood Transfusions Safe: FDA’s Multi-layered Protections
for Donated Blood. 2002; Publication No. FS 02-http://www.fda.gov/opacom/factsheets/justthefacts/15blood.html
. Accessed December 20, 2007. - Price TH, ed. Requirements for storage, transport and expiration.
In Standard for blood bank and transfusion services. 25th ed.
Bethesda, MD: AABB, 2008. 63: 5.1.8A #7. - Whitaker BI, Henry R. National Blood Collection and Utilization
Survey Report. 2005; National Blood Data Resource Center, US
Department of Health and Human Services, Washington, DC. - Scholz, PM, Karis JH, Gump FE, Kinney JM, Chien S. Correlation of
blood rheology with vascular resistance in critically ill patients.
Journal of Applied Physiology. 1975; 39(6):1008-1011. - Kosh CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T,
Blackstone EH. Duration of red-cell storage and complications after
cardiac surgery. NEJM. 2008;358(12):1228-1239. - Ignaro LJ. No More Heart Disease. 2005. St. Mathews Press.
NY, NY. - Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red
blood cells in sickle cell disease. Proceedings of the National
Academy of Science (PNAS). 2005; 102(7): 2531-2536. - Valeri CR, Hirsch NM. Restoration in vivo of erythrocyte adenosine
triphosphate, 2,3 diphosphoglycerate, potassium ion and sodium ion
concentrations following the transfusion of acid-dextrose-stored human
red blood cells. J Lab Clin Med. 1969;73(5):722-733. - Bonaventura, J. Clinical implications of the loss of vasoactive
nitric oxide during red blood cell storage. Proceedings of the
National Academy of Science (PNAS). 2007; 104 (49): 19165- 19166 - Stamler JS, Jia I, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J,
Gernet K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in
the physiological oxygen gradient. Science - Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin:
a dynamic activity of blood involved in vascular control. Nature. 1996;380:221-226.