Life sustains due to all living beings’ ability to procreate. The primal need to procreate is as basic as our need to breathe to survive. The health of the human species lies, in a big part in its ability to co-exist with a plethora of micro-organisms that are present in the environment and with the microorganisms that inhabit it. In a healthy state, the human body is in equilibrium with the microorganisms that it houses. However, when immunocompromised, these very organisms can have deleterious effects on the host. A report by the panel of the National Academy of Public Administration for the National Coalition of STD directors (NCSD) commented on the impact of Sexually Transmitted Infections (STIs) in the United States1 and proposed a national strategy to control the epidemic of STIs. The most common STIs are herpes simplex virus (HSV), human papillomavirus (HPV), Trichomonas vaginalis (TV), Chlamydia trachomatis (CT), Neisseria gonorrhea (NG), hepatitis virus (HBV, HCV), human immunodeficiency virus (HIV), Mycoplasma genitalium (MG), and syphilis (Treponema pallidum).2
Rising epidemic of STIs
STIs, including chlamydia, gonorrhea, and syphilis were on the decline until 2009, with the most recent surveillance data reported by CDC from 2017,2 showing a marked increase across the different STIs. The U.S. now leads developed countries in the rate of increase of STIs. Astounding numbers define this increase, with congenital syphilis at an increase of 154 percent, gonorrhea at 67 percent, and chlamydia at 22 percent.2(Figure 1) Chlamydia is now the most common STI in the U.S. with 1.7 million cases reported in 2017. The burden of STIs are borne by adolescents, young people (15-24 years), gay and/or bisexual men, and pregnant women. The true numbers of STIs must far outnumber current estimates as these infections are mostly asymptomatic as noted by an Institute of Medicine3 report, The Hidden Epidemic: Confronting Sexually Transmitted Diseases.Globally, STIs are still diagnosed by symptoms; a syndrome is based on a set of criteria that assumes the presence of (a) specific pathogen(s). The symptom(s), therefore direct the prescription and initiation of treatment at the initial visit, instead of the diagnostic test results. The most common symptoms observed are urethritis, cervicitis, epididymitis, pelvic inflammatory disease (PID), vaginal discharge, papular anal/genital lesions, genital ulcers, and intestinal and enteric syndromes (proctitis, proctocolitis, enteritis). The pathogens responsible for these symptoms could be CT, NG, TV, or MG.
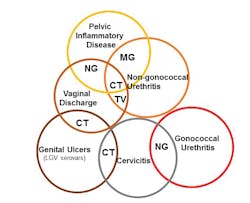
(Figure 2)Trichomonas vaginalis is often described as the “forgotten STI” and causes the most non-viral STIs. TV infection rates are not reported, as it is still referred to as a nuisance infection. However, the implications of having a persistent TV infection can have a significant impact on overall health. The World Health Organization (WHO) estimates new TV infections at 143 million annually. The incidence of MG infections and effects on health are being reported causing the Centers for Disease Control and Prevention (CDC)4 to classify it as an emerging pathogen with some experts postulating it to be the first superbug, with alarming rates of resistance observed globally.The increased resistance of NG and MG to available treatment options have resulted in various initiatives in the U.S. and across the globe to improve diagnostics and treatment. The STI Clinical Trials Group5 (STI CTG) aims to foster, develop, and administer research programs that specifically lead to the prevention and control of STIs. The WHO has now proposed a global health sector strategy for the prevention and control of STIs.6 The strategy targets a reduction of 90 percent of NG and T. pallidum incidence globally by 2030. The control of STIs, while seemingly an arduous task, can be accomplished via education, prevention, accurate diagnosis, and treatment. The healthy people7 initiative developed by the Office of Disease Prevention and Health Promotion is a step in the right direction to assist in the dissemination of information about prevention of STIs with evidence-based interventions and resources. One of the oldest organizations involved in the effort of education, study, and control of STIs is the American Sexually Transmitted Diseases Association.8 ASTDA has been long involved with education efforts with the latest being an implementation of an online resource: stdpreventiononline.org.9
Prevention of STIs
Education and prevention are key pillars in the effort of controlling the STI epidemic. While this effort is vital, it must be fortified by accurate diagnostics and treatment. Prior to the advent of polymerase chain reaction (PCR) technology, microbiological culture methods were the preferred method of diagnostics. Following Koch’s postulates, cultures were/are deemed in many cases as the true test for isolating a pathogen and implication as the causative agent of disease. Diagnostic culture tests served the clinical community for quite some time. However, microbiological culturing is time consuming as laboratories and diagnostic providers must culture the pathogen in an appropriate growth medium. Therefore, diagnostic culture tests take time and require skilled microbiologists to conduct tests and report accurate results.
The need for better diagnostics led to the introduction of ligase chain reaction test10 for detection of chlamydia with better sensitivities and specificities than culture or direct fluorescent-antibody assays. The advent of nucleic acid amplification tests (NAAT) with its high specificity and sensitivity brought about remarkable changes in the clinical diagnostic space. The introduction of PCR based CT/NG testing, along with screening guidelines to test for infections, brought a paradigm shift in the diagnostic space. The testing times were dramatically reduced, with results available in a few days instead of weeks. Screening also allowed the identification of asymptomatic infected individuals, which in the case of STIs, is the clear majority of infections.
From necessity sprang the creation of a whole new generation of diagnostic tests now classified as point of care (POC).11 These diagnostic tests do not need to be done in traditional hospital laboratories and can be housed in clinics and near-patient settings. The POC tests allow for rapid test results often under an hour albeit without the remarkable specificity and sensitivity of NAATs. POC testing is advantageous as it allows for the rapid identification of the causative organism, especially in case of syphilis. In addition, this is a great tool in settings where the risk of losing patients to follow up is high. However, most POC testing modalities are restricted to a single test. Complex STIs, which could have multiple causative agents, could be a potential hurdle for POC testing. POC tests are designed for near-patient settings, which limits the ability to do large volume testing as compared to the traditional laboratory instruments. From an operational perspective, a lab would benefit from minimal interactions with the large number of samples in a national screening/diagnostic program.
STI screening and testing
A national screening program for STIs like CT, NG, TV, MG, or HPV would involve large numbers of samples. Testing for STIs is a challenge for diagnostic manufacturers for a variety of reasons, at the forefront is the myriad of sample types that could be utilized. The sample types differ with respect to gender and anatomical sites. Furthermore, for certain STIs, there is a lack of consensus on the appropriate sample type that can/should be used in a screening assay. Screening/testing urogenital specimens for CT/NG/MG could result in missing many infections as a significant percent are rectal and oral infections, especially in high-risk populations. There are no FDA-approved tests for testing extra-genital specimens, however, there are multiple validated lab developed tests (LDTs). Diagnostic test providers have specific swabs for collecting specimens from the vagina, endocervix, throat, and a separate collection device for urine. For cervical cancer screening, liquid-based cytology (LBC) devices utilizing a cervical brush or broom is preferred. Laboratories must train healthcare professionals on different collection devices to ensure the appropriate device is used to collect specimens for the various assays. This obviously brings logistical challenges, considering the number of specimens that need to be collected.
Self-collection specimens
In recent years, screening programs have realized the importance of including individuals that do not turn up for screening or are unresponsive to invitations for testing. The solution to this challenge seems to have an option for specimen collection at home—wherein, individuals receive specimen collection kits at their residence, they self-collect specimens and mail it to the testing site. Evidence from various studies12 indicate that individuals are receptive to this approach and it could include individuals unresponsive to participation in the screening program. The singular collection device would be ideal for specimens collected at home and healthcare premises. The universal collection device would make collections and testing seamless for laboratories, with less specimens rejected due to incorrect specimen collection. Considering the large numbers of specimens collected in this universal collection device, specimens after accessioning and without additional processing could be placed on the diagnostic instrument. The instrument would be able to sample through a pierceable cap for testing and report results.
STI assay development challenges
STI assays would be the next logistical challenge for laboratories and physicians. One could consider an assay that could test for the most common STIs that have the highest incidence. Rather than an all-encompassing microbial panel the assay would be specific to the microbes that cause the most harm (e.g. CT, NG, TV, MG). This assay combination with chlamydia (the most common STI), TV (that has a prevalence of 2.3 million in women aged 14-49)2, NG (which saw an increase of 67 percent), and MG (the second most common cause of nongonococcal urethritis).13,14 Testing for these STIs in a single test and rapid reporting of results could enable a physician to instill a treatment program based on etiology rather than observed symptom(s). If the physicians could get results in a time frame that allowed the individual to wait for the correct prescription, it would not only ensure that appropriate treatment is provided, but also open the conversation for partner notification and treatment. This is a vital cog in the wheel to prevent the of spread of infection, as partner notifications tend to get lost in the testing algorithm or occurs later in the process. This delay in partner treatment is part of the reason why STIs continue to spread.
An additional factor would be offering physicians the option for testing any combinations of the STIs mentioned, as appropriate to their geographical locations. Considering the symptoms manifested by CT, NG, TV, and MG are overlapping, it would be pertinent to have an assay that identifies the causative organism(s). Additionally, labs could contribute to epidemiological surveillance by identifying increased incidence rates of an infection in real time, a function that public health labs are required to do. In the age of big data, this could be handled by diagnostic instruments with software features providing reports on infection rates for set time periods. This would enable each lab testing for STIs to provide real time incidence rates from their geographical locations thereby enabling a faster response by public health agencies.
HPV
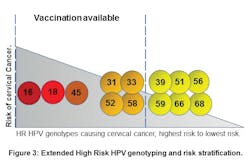
An infection that disproportionally affects the female population is HPV. Cervical cancer ranks as the fourth most common type of cancer, with anal, oropharyngeal cancer incidence due to HPV on the increase. Pap smear was the diagnostic test employed to identify individuals with cellular abnormalities due to cervical cancer. This testing modality evolved to LBC due to advantages over the traditional pap smear. The introduction of molecular HPV tests with identification of high risk (HR) HPV genotypes provided added information that enabled physicians to triage women based on the presence or absence of 16/18 HR HPV genotypes. These two HR HPV genotypes are responsible for about 70 percent of cervical cancer cases. Evidence in recent years points to a screening modality that identifies the HR HPV genotype persistent in an individual, thereby driving a monitoring program that is based on the HR HPV genotype detected. The approach of risk-stratifying individuals15 based on the HR HPV genotype (Figure 3) would preclude the increase of individuals (who are detected with HR HPV genotypes with low incidence of causing cancer) being sent to colposcopy. Considering requirements for home-collect specimen collection devices and the need to empower all women to participate in a screening program, the molecular HR HPV assay would have built-in controls to ensure that the sampling was adequately done. With most of the sexually active population participating in the screening/testing program, appropriate assay controls need to be incorporated to ensure testing laboratories have the required confidence in the results that are generated.Clinical laboratory challenges
Laboratories faced with burgeoning healthcare costs, increase in testing volumes, and a dearth of skilled technologists are challenged with cost cutting measures and ensuring efficiency in their operations. Embarking on an STI screening/testing program can be daunting, even with the current generation of batch-based instruments or the newer “random” access instruments. The clinical community needs an instrument that accommodates testing any combination of assays and/or any array of STI tests. Such an instrument should be able to accept samples with minimal processing after entering the laboratory workflow. Technologists should be able to load and go, both for reagents and samples, with an intuitive user interface that allows easy use by current and experienced generations of technologists. The diagnostic instrument should be able to accept specimens and reagents continuously, accommodating a laboratory’s workflow and needs; as opposed to current instruments where workflows are accommodated based on the capability of an instrument to process samples.
Conclusion
The current status of STIs can be described as an epidemic. This comes on the back drop of cuts in healthcare, consolidation of laboratories, and closure of STD clinics. Diagnostic technology has improved vastly with microbiological culture and biochemical tests being replaced or supplemented with molecular tests. Despite all the developments in the past few years, STIs are still on the rise. This calls for an increased effort from individuals engaged in healthcare and society as a whole to take the mantle of education and prevention. On an operational basis, the introduction of a universal sample collection device, multiplex STI assay, HR HPV assays with extended genotyping, and a true random, continuous access instrument should be able to transform greater than 50 percent of all testing done in laboratories. While diagnostic providers and healthcare professionals will support the fight against STIs in the clinical space, education and prevention will help decrease the onslaught of STIs, helping more people experience a sexually healthy life.
REFERENCES
- National Coalition of STD Directors. The impact of sexually transmitted diseases on the United States: Still hidden, getting worse, can be controlled. December 2018.
- Sexually Transmitted Disease Surveillance 2017. Centers for Disease Control and Prevention. National center for HIV/AIDS, Viral Hepatitis, STD and TB prevention, Division of STD prevention. September 2018.
- The hidden epidemic: confronting sexually transmitted diseases. Institute of Medicine, Washington, DC: National Academy Press. (1997)
- WHO. Sexually Transmitted Infections (STIs). https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis). Accessed February 26, 2019.
- STAR Sexually Transmitted Infections Clinical Trials Group. https://starstictg.s-3.net/. Accessed February 26, 2019.
- Global Health Sector Strategy on Sexually Transmitted Infections, 2016-2021. WHO, July 2016.
- Office of Disease Prevention and Health Promotion. Sexually Transmitted Diseases. https://www.healthypeople.gov/2020/topics-objectives/topic/sexually-transmitted-diseases. Accessed on February 26, 2019.
- American Sexually Transmitted Diseases Association. https://www.astda.org/. Accessed February 26, 2019.
- http://www.stdpreventiononline.org/. Accessed February 26, 2019.
- Schachter J, Stamm EW, Quinn CT, et al. Ligase chain reaction to detect chlamydia trachomatis infection of the cervix. J. Clin. Micro. 1994; 32(10):2540-2543.
- WHO, The point of care diagnostic landscape for sexually transmitted infections (STIs). https://www.who.int/reproductivehealth/topics/rtis/Diagnostic_Landscape_2018.pdf. Accessed February 26, 2019.
- Snijders JFP, Verhoef MJV, Arbyn M, et al. High-risk HPV testing on self-samples versus clinician-collected specimens: A review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int. Jr of Cancer. 2013; 132(10): 2223-2236.
- Martin HD, Manhart EL, Workowski AK. Mycoplasma genitalium from basic science to public health: Summary of the results from a NIAID technical consultation and consensus recommendations for future research priorities. JID. 2017;216 (Suppl 2):S427-430.
- Gaydos C, Maldeis EN, Hardick A, et al. Mycoplasma genitalium compared to Chlamydia, Gonorrhea and Trichomonas as an etiologic agent of urethritis in men attending STD clinics. Sex Transm. Infect. 2009;85(6):438-440
- Schiffman M, Wentzensen N, Khan JM, et. al. Preparing for the next round of ASCCP-sponsored cervical screening and management guidelines. J. Low Genit Tract Dis. 201;21(2):87-90.
About the Author