Group B streptococcus: Beyond pregnancy and neonatal infections
Streptococcus agalactiae, also known as Group B streptococcus (GBS), is a gram-positive coccus that was first differentiated from other beta-hemolytic streptococcal species by renowned microbiologist Rebecca Lancefield, PhD, in the early 1930s.1 Her seminal work established the Lancefield grouping based on an immunologic reaction of carbohydrate antigens expressed on the bacterial cell wall. Serogrouping of the C-carbohydrate expressed by beta-hemolytic streptococci has remained useful for rapid identification and patient management even in the era of advanced identification methods, e.g., matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF).
Upon its first recognition in 1917, GBS has remained a significant cause of bovine mastitis in cattle.2 Despite being initially regarded as an animal pathogen, it can cause a variety of infections in human hosts. GBS easily colonizes the genitourinary and gastrointestinal tracts in humans. It can also colonize the upper respiratory tract, but to a lesser extent.
In 1996, the Centers for Disease Control and Prevention (CDC), in collaboration with key stakeholders and several professional societies published the first guidelines on preventing GBS disease. These guidelines were subsequently updated in 2002 and again in 2010.3 As a result of screening and intervention, the incidence of GBS disease declined substantially.4 In 2019, the CDC assigned ownership of guideline components to three professional organizations: The American Academy of Obstetrics and Gynecology (ACOG) and the American Academy of Pediatrics are responsible for guidelines related to the prophylaxis and treatment of GBS in pregnant women and newborns, respectively, while the American Society for Microbiology (ASM) is charged with updating guidelines and best-practices related to the detection and identification of GBS.5-7
Epidemiology of Group B streptococcal infections in the United States
In many state public health jurisdictions, reporting of GBS infections is not required. Beginning in the late 1990s, the CDC began active surveillance for GBS through the Active Bacterial Core surveillance (ABCs) network.8 The ABCs is a multistate, population-based surveillance system for invasive bacterial pathogens, including GBS. The ABCs are an integral component of the Emerging Infections Program9 within the Division of Preparedness and Emerging Infections. This program spans six states and four multi-county areas in additional states covering a population of nearly 45 million people.
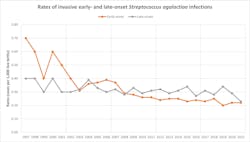
Since the ABCs began tracking GBS in 1997, the overall rate of GBS has increased 42% from 6.2 per 100,000 to 8.8 per 100,000 in 2021, the last year of data currently available. Despite the increase in number of cases, the mortality rate has remained stable during this time period (Figure 1).Group B streptococcal infection in pregnant women
GBS can manifest in a variety of clinical syndromes in pregnant women, including urinary tract infections, post-operative caesarean wound infections, endometritis, and bacteremia. Fulminant infections, including meningitis and endocarditis have also been reported, though these are rare.
Urinary tract infections are most commonly reported during pregnancy. GBS can cause uncomplicated cystitis and pyelonephritis. Asymptomatic colonization with GBS during pregnancy is a risk factor for later infections as well as infections in the neonate following birth. In one retrospective cohort study, untreated GBS bacteriuria had an odds ratio of 7.2 (95% confidence interval 2.4 to 21.2) for developing chorioamnionitis, and increasing colony counts of GBS reported in culture was also associated with increasing grade of chorioamnionitis.10
Other complications of GBS carriage during pregnancy includes endometritis and bacteremia. One retrospective cross-sectional study of more than 7,922 pregnant women indicated that postpartum endometritis was 1.8 times more likely in women colonized with GBS.11 Bacteremia due to GBS remains a concern in peripartum individuals. In a large retrospective cohort from 2009–2016, GBS was isolated from positive blood cultures in 11% of cases.12 Other infections may include meningitis, endocarditis, abdominal abscess, and necrotizing fasciitis, though these are rare peripartum complications.
Group B streptococcal infection in neonates
In the 1970s, GBS emerged as the leading cause of morbidity and mortality among neonates. In the United States, the reported case fatality rates reached nearly 50%.13,14 GBS in neonates is acquired in utero resulting from intraamniotic infection or rupture of membranes, as well during birth as a result of passing through the vagina. Worldwide, the reported incident rate of GBS is 0.5 per 1,000 live births.15
GBS disease can be classified based on disease onset as either early-onset or late-onset disease. Combined, the incidence rate for GBS disease is approximately 0.5 per 1,000 live births (Figure 2). Early-onset disease of GBS is defined as the identification and/or isolation of S. agalactiae from blood, cerebrospinal fluid (CSF), or another sterile site from birth through six days of age.16 More than 95% of early-onset GBS and 97% of late-onset GBS in the United States are due to serotypes Ia, Ib, II, III, IV, and V.17 Neonates that develop early-onset GBS disease typically present with sepsis (80-85% of cases), pneumonia (approximately 10% of cases), and meningitis (5-10% of cases).Late-onset GBS disease is defined as disease occurring at four to five weeks of age. Patients typically present with bacteremia (65% of cases) and meningitis (25% to 35% of cases). Late-onset disease can be further broken down into late, late-onset GBS disease, which occurs in infants older than three months of age. Typically, infants who develop late, late-onset GBS were born pre-term before 28 weeks of gestation or have a history of immunodeficiency.18,19 Other manifestations of late-onset disease can include pneumonia, septic arthritis, and osteomyelitis.20
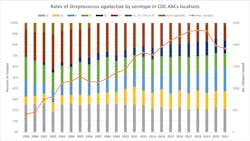
The ABCs surveillance network also performs additional characterization of GBS isolates from selected surveillance areas using whole genome sequencing. Performing whole genome sequencing allows epidemiologists to determine capsular serotypes, multi-locus sequence typing, and phylogenetic clustering to identify transmission events. During the 22 years with data available, serotypes for 32,808 isolates have been determined. There are slight variations in the proportion of serotypes year over year, but notably serotype Ia has remained fairly stable, representing approximately 20% of isolates each year of the program. Some serotypes have seen increases over time (serotype II) whereas others have decreased in recent years (serotype V, non-typable isolates) (Figure 3).Group B streptococcal infection in nonpregnant persons
Infections due to GBS in nonpregnant persons is high. In the United States, the incidence among all persons was estimated to be 11 cases per 100,000 persons in 2016.21 The incidence for GBS is higher among older adults.22 Some studies have tried to identify specific risk factors for GBS infection. Diabetes is a commonly reported underlying medical condition in patients with GBS disease.
A variety of infection types have been described in the medical literature from retrospective cohort and case-control studies. Many times, cases of GBS in nonpregnant persons are classified as nosocomial. Skin and soft tissue infections, bacteremia, urinary tract infections, pneumonia, meningitis, septic arthritis, endocarditis have all been described in patients. Therefore, isolation and identification of GBS from any normally sterile site is significant and should be reported.
Laboratory surveillance for GBS
Laboratory testing remains a crucial component for rapid detection of GBS maternal colonization. The ACOG recommends performing universal GBS screening between 36 0/7 and 37 6/7 weeks of gestation. All women whose vaginal–rectal cultures at 36 0/7–37 6/7 weeks of gestation are positive should receive appropriate intrapartum antibiotic prophylaxis.3 Cultures for GBS most accurately predict colonization status at birth if done within five weeks prior to delivery. Changing the interval from 35–37 weeks to 36 0/7 as the baseline was done to ensure more accurate results for the nearly 7% of births that occur after 31 weeks of gestation.23
It is recommended to use a single-flocked swab and obtain a specimen first from the lower vagina and then from the rectum without the use of a speculum. Use of a single swab to sample both anatomic locations has been shown to increase sensitivity for detecting GBS.24 Flocked swabs release microorganisms more effectively, thereby increasing GBS detection and recovery compared to traditional fiber swabs.25 Specimens should be transported to the laboratory and processed within 24 hours of collection, especially when culture methods are used. Amies transport medium or ESwab transport systems are preferred over sending a dry swab to the lab in a sterile container. Additionally, it is recommended to hold specimens refrigerated at 4–8°C if there is a delay in sending to the testing laboratory. Culturing specimens greater than 24 hours may yield a false-negative result. Specimens not processed within 24 hours should be rejected and recollection is recommended.
Testing for GBS colonization can be done by culture method or by using nucleic acid amplification testing (NAAT), e.g., PCR. Direct plating of swabs onto culture media may be performed in some labs, to reduce time to detection, but it should not be the sole means of screening for GBS carriage. Use of liquid broth media is recommended, and may include nonselective, selective, and differential broth media types to aid in the recovery of GBS. Nonselective broths, when used, can overgrow other vaginal or gastrointestinal microbiota making detection a challenge. Selective media can result in a 2.5-fold increase in detection of GBS compared to use of non-selective broth.26 Some differential broths, such as Carrot Broth, support pigment production by hemolytic GBS strains. When positive, these samples can be reliably reported based on pigment observation. The pigment production is highly specific for presence of GBS and is sensitive for hemolytic strains. However, non-beta-hemolytic GBS strains are not detected by this method.27 Sub-culture of pigment-negative broth culture is still recommended.
Several NAAT test manufacturers are approved for testing following a broth enrichment step and do not require culture confirmation of negative results. The main limitation of NAAT-based testing approaches is the lack of recovery of an isolate for antimicrobial susceptibility testing. The recommended intrapartum antimicrobial prophylaxis is penicillin or cefazolin; therefore, AST is warranted for women who have a documented penicillin allergy. Though clindamycin is the recommended alternative,3 rates of clindamycin resistance in GBS have been increasing from 20.2% in 2006 to 49.1% in 2021 based on data from the CDC.8
Conclusions regarding GBS disease and surveillance testing
Even though recommendations regarding testing and administration of intrapartum antimicrobial prophylaxis have been in use for more than 25 years, neonatal disease due to GBS remains a significant cause of morbidity and mortality. Clinical microbiology laboratories are required to perform screening for GBS among pregnant women. The timing of when screening cultures should be collected has evolved, in part due to the awareness that colonization with GBS can be intermittent. Performing NAAT following a broth enrichment step increases sensitivity for GBS detection. Though culture remains an important component for ensuring appropriate antimicrobial agent selection among women with a penicillin allergy.
References
1. Lancefield RC. A Serological Differentiation of Human and Other Groups of Hemolytic Streptococci. J Exp Med. 1933;31;57(4):571-95. doi:10.1084/jem.57.4.571.
2. Keefe G. Update on control of Staphylococcus aureus and Streptococcus agalactiae for management of mastitis. Vet Clin North Am Food Anim Pract. 2012;28(2):203-16. doi:10.1016/j.cvfa.2012.03.010.
3. Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;19;59(RR-10):1-36.
4. Schrag SJ, Zywicki S, Farley MM, Reingold AL, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;6;342(1):15-20. doi:10.1056/NEJM200001063420103.
5. Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obstet Gynecol. 2020;135(2):e51-e72. doi:10.1097/AOG.0000000000003668. Erratum in: Obstet Gynecol. 2020 Apr;135(4):978-979.
6. Puopolo KM, Lynfield R, Cummings JJ; COMMITTEE ON FETUS AND NEWBORN; COMMITTEE ON INFECTIOUS DISEASES. Management of Infants at Risk for Group B Streptococcal Disease. Pediatrics. 2019;144(2):e20191881. doi:10.1542/peds.2019-1881.
7. Asm.org. Accessed May 26, 2023. https://asm.org/Guideline/Guidelines-for-the-Detection-and-Identification-of.
8. Active Bacterial Core surveillance system (ABCs). Cdc.gov. Published July 19, 2021. Accessed May 26, 2023. https://www.cdc.gov/abcs/index.html.
9. Emerging Infections Program. Cdc.gov. Published May 17, 2023. Accessed May 26, 2023. https://www.cdc.gov/ncezid/dpei/eip/index.html.
10. Anderson BL, Simhan HN, Simons KM, Wiesenfeld HC. Untreated asymptomatic group B streptococcal bacteriuria early in pregnancy and chorioamnionitis at delivery. Am J Obstet Gynecol. 2007;196(6):524.e1-5. doi:10.1016/j.ajog.2007.01.006.
11. Krohn MA, Hillier SL, Baker CJ. Maternal peripartum complications associated with vaginal group B streptococci colonization. J Infect Dis. 1999;179(6):1410-5. doi:10.1086/314756.
12. Wilkie GL, Prabhu M, Ona S, Easter SR, et al. Microbiology and Antibiotic Resistance in Peripartum Bacteremia. Obstet Gynecol. 2019;133(2):269-275. doi:10.1097/AOG.0000000000003055.
13. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;25;230(8):1158-60.
14. Horn KA, Meyer WT, Wyrick BC, Zimmerman RA. Group B streptococcal neonatal infection. JAMA. 1974;25;230(8):1165-7.
15. Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, et al. Infant Group B Streptococcal Disease Incidence and Serotypes Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. 2017;6;65(suppl_2):S160-S172. doi:10.1093/cid/cix656.
16. American Academy of Pediatrics. Group B streptococcal infections. Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL: American Academy of Pediatrics; 2018:762–768.
17. Nanduri SA, Petit S, Smelser C, Apostol M, et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019;1;173(3):224-233. doi:10.1001/jamapediatrics.2018.4826.
18. Hussain SM, Luedtke GS, Baker CJ, Schlievert PM, Leggiadro RJ. Invasive group B streptococcal disease in children beyond early infancy. Pediatr Infect Dis J. 1995;14(4):278-81. doi:10.1097/00006454-199504000-00006.
19. Guilbert J, Levy C, Cohen R; Bacterial meningitis group; Delacourt C, Renolleau S, Flamant C. Late and ultra late onset Streptococcus B meningitis: clinical and bacteriological data over 6 years in France. Acta Paediatr. 2010;99(1):47-51. doi:10.1111/j.1651-2227.2009.01510.x.
20. Berardi A, Rossi C, Lugli L, Creti R, et al. Group B streptococcus late-onset disease: 2003-2010. Pediatrics. 2013;131(2):e361-8. doi:10.1542/peds.2012-1231.
21. Francois Watkins LK, McGee L, Schrag SJ, Beall B, et al. Epidemiology of Invasive Group B Streptococcal Infections Among Nonpregnant Adults in the United States, 2008-2016. JAMA Intern Med. 2019;1;179(4):479-488. doi:10.1001/jamainternmed.2018.7269.
22. Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008;7;299(17):2056-65. doi:10.1001/jama.299.17.2056.
23. Martin JA, Osterman MJ, Kirmeyer SE, Gregory EC. Measuring Gestational Age in Vital Statistics Data: Transitioning to the Obstetric Estimate. Natl Vital Stat Rep. 2015;1;64(5):1-20.
24. El Aila NA, Tency I, Claeys G, Saerens B, et al. Comparison of different sampling techniques and of different culture methods for detection of group B streptococcus carriage in pregnant women. BMC Infect Dis. 2010;29;10:285. doi:10.1186/1471-2334-10-285.
25. Silbert S, Rocchetti TT, Gostnell A, Kubasek C, Widen R. Detection of Group B Streptococcus Directly from Collected ESwab Samples by Use of the BD Max GBS Assay. J Clin Microbiol. 2016;54(6):1660-1663. doi:10.1128/JCM.00445-16.
26. Baker CJ, Clark DJ, Barrett FF. Selective broth medium for isolation of group B streptococci. Appl Microbiol. 1973;26(6):884-5. doi:10.1128/am.26.6.884-885.1973.
27. Church DL, Baxter H, Lloyd T, Miller B, Elsayed S. Evaluation of StrepB carrot broth versus Lim broth for detection of group B Streptococcus colonization status of near-term pregnant women. J Clin Microbiol. 2008;46(8):2780-2. doi:10.1128/JCM.00557-08.
About the Author

Nicholas M. Moore, PhD, D(ABMM), MLS(ASCP)cm
is the Associate Director of Clinical Microbiology and Associate Professor, Rush University Medical Center in Chicago, Illinois. His research interests are focused on antimicrobial resistance in Gram-negative bacilli, and the use of advanced molecular diagnostics to inform infection control decision-making related to nosocomial outbreaks.