Can medical laboratories give humanity the edge over tuberculosis?
To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Discuss healthcare statistics and the causative agent of Tuberculosis (TB).
2. Describe the differences in documented TB infections.
3. Describe detection methods and types of assays for TB and their benefits and limitations.
4. Discuss the current recommended protocols for the identification and diagnosis of TB.
Tuberculosis (TB) disease is both preventable and curable, and yet — much to the surprise of many who mistakenly consider its threat extinguished or unremarkable — TB was the 13th leading cause of death worldwide in 2021.1 Among infectious disease killers, the World Health Organization has ranked it the top infectious disease killer, second only recently to COVID-19.
And in much the same way laboratory testing and analysis have played a critical role in diagnosing, reporting, and monitoring COVID-19 — and informing treatment decisions — so too can the laboratory community play a crucial role in stopping TB.
A brief snapshot of TB history
TB is not a new disease. It can be traced back 9,000 years where it was found in the human remains of a mother and child buried together in a city now submerged beneath the Mediterranean Sea. The earliest written mentions of TB were in India 3,300 years ago and in China 2,300 years ago. Between the 1600–1800s in Europe, TB caused 25% of all deaths, with a similar impact in the United States. The New York City Department of Health and Hygiene published its first report on TB in the city in 1893. On March 24, 1882, Dr. Robert Koch announced the discovery of the bacterium that causes TB. Each year, public health agencies and organizations around the world mark World TB Day on March 24 to raise public awareness about the global TB epidemic.
Today, an estimated one quarter of the world’s population is infected with a latent TB infection (LTBI), and in 2021 an estimated 10.6 million people around the world became actively sick with the disease, including 6 million men, 3.4 million women, and 1.2 million children.3 Every year, about 1.5 million people die from TB all over the world, and while a majority live in low- and middle-income countries, TB is everywhere. In the United States, the Centers for Disease Control and Prevention (CDC) says an estimated 13 million Americans have LTBI and 7,882 active cases of the disease were reported in 2021.3
Understanding TB
Highly contagious, TB is an airborne infectious disease caused by Mycobacterium tuberculosis (MTB). It usually affects the lungs but can also impact other parts of the body such as the kidneys, spine, or brain.6
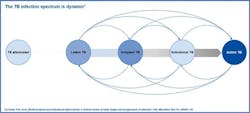
A TB infection historically had two general states — latent TB infection (LTBI) and active TB disease. Recent research has demonstrated that human TB infection, from LTBI to active TB disease, exists within a continuous spectrum of metabolic bacterial activity with antagonistic immunological responses. This paradigm shift in thinking has led to the proposal of two additional clinical states: incipient and subclinical TB.6 See Figure 1.
When incipient and subclinical TB are identified, latent and active TB cases can be divided along the clinical disease spectrum, providing opportunities for diagnostic and therapeutic interventions to prevent progression to active TB disease and transmission of TB bacilli. Therefore, not everyone infected with TB bacteria progresses to an active TB infection but can be somewhere within the spectrum of TB. Without treatment, LTBI can progress to TB disease. But both can be treated. LTBI regimens generally take three to four months to complete, although some protocols can take up to nine months.7 TB disease regimens generally take from four to nine months to complete. Drug resistant TB is more difficult and costly to treat and regimens may take up to two years.
Screening, accurately diagnosing, and treating LTBI have become a focal point of global efforts to end TB.9 LTBI causes no symptoms or discomfort and is not contagious so most infected people are unaware of their condition. But unless it is treated, one in ten people with LTBI will become ill with TB disease in the future, according to the CDC.
The risk is elevated for people with HIV, diabetes, or other conditions, and for those on treatments that affect the immune system. In fact, TB is the leading cause of death among the 38.4 million people living with HIV.9
Clearly medical science, public health agencies and care delivery professionals have made incredible strides to diminish TB’s impact on humans. Due to diagnosis and treatment of both LTBI and TB disease, the CDC estimates that more than 66 million lives were saved between 2000 and 2020.9 But much more needs to be done.
Ending TB through better diagnostics
Globally, world health leaders are working toward TB elimination by 2035.10 The effort is multi-faceted and involves more than 25 countries. The core of this work is to find and treat latent TB through better screening, contact tracing, and diagnostics, including providing training and technical support to scale use of new and faster diagnostic tools.
Without question, today’s laboratories have a growing role to play in support of newer, more specific, less subjective, and faster testing solutions and can spur use of new diagnostic tools over outdated skin testing techniques.
The Mantoux tuberculin skin test
Tuberculin skin tests (TSTs) date back more than 100 years. The tine test, a multi-pronged tuberculin skin test was used for about a century but was abandoned in about 2000 in favor of the Mantoux test. Still in use today, the Mantoux test is generally administered in a physician’s office or, more recently, in occupational health settings, and even pharmacy-based walk-in clinics. This test is not done in the laboratory setting.
The Mantoux test is a delayed-type hypersensitivity reaction used to detect if a patient is infected with M. tuberculosis. The skin test involves intradermal administration of tuberculin units of purified proteins (PPD) solution. A follow-up visit is required within 48 to 72 hours so the results can be interpreted in the office. The reading of the test is subjective and therefore the experience level of the reader can affect the results.
The results record the induration as the reaction to the PPD in millimeters (mm) by measuring the induration. The TST can require up to four patients visits, which correlates to a high “no-show” rate. The test then must be redone with another follow-up visit. Additionally, the results of the TST test can be affected by the Bacille Calmette-Guérin (BCG) vaccination—a vaccine for TB. TST can have a specificity as low as 59% in BCG-vaccinated patients translating to an increase in false positive results from cross reaction with patients who have had the BCG vaccination.
The interferon gamma release assays
Interferon gamma release assays (IGRAs) are tests that measure the immune response to TB proteins to determine if a patient is infected with Mycobacterium tuberculosis. These tests are conducted and analyzed in a laboratory setting from a blood sample instead of a primary care or other clinical settings. For specific patient populations, the CDC encourages their use over TSTs.11
IGRAs provide many benefits for both clinicians and patients. IGRAs are a single-visit screening test, they are highly accurate, and they have reproducible results. It is an objective, lab-based test in comparison to the TST which is a subjective test.
Two IGRAs are approved by the U.S. Food and Drug Administration (FDA): QuantiFERON-TB Gold Plus (QFT-Plus) and T-SPOT.TB.
Both IGRAs measure secretion of cytokine interferon gamma (IFN-g) as a marker of cell-mediated immune response to TB-specific peptides. They also elicit both a CD8 and CD4 T-cell response, and in the case of QFT-Plus, the response attributed to each cell type can be approximated, which allows for a comprehensive assessment of cell-mediated immune response to TB infection. This interferon gamma is measurable, stable, and typically absent from normal circulation.
QuantiFERON-TB Gold Plus (QFT-Plus) is a whole blood stimulation followed by ELISA or Chemiluminescent detection of IFN-g. In the registration trials and publications by Barcellini, et al12-14 on QFT-Plus, the isolated CD8 response was calculated by subtracting the quantitative values of TB1 from TB2 and potentially found to be enhanced in the following conditions:
- Frequently in active untreated pulmonary tuberculosis
- Among some persons with higher risk for TB exposure
- Among some persons recently exposed to active TB
- Among some contacts who had higher association to cumulative exposure and being European born (as opposed to being born in higher burden settings)
Today’s recommended test protocols
Both TST and IGRA tests are approved for use in the United States, and both are generally covered by Medicare, Medicaid, and private insurance plans.
The Infectious Disease Society of America recommends IGRA tests rather than TSTs in individuals five years or older who meet the following criteria:
- They are likely to be infected with MTB
- They have a low or intermediate risk of disease progression
- It has been decided that testing for LTBI is warranted
- And they either have had a BCG vaccination or they are unlikely to return to have their TST read (strong recommendation, moderate-quality evidence)15
The TST is recommended by the CDC for children under the age of five, primarily because blood tests can be more difficult for young children, however, IGRAs are approved for use with children under five years old.
Who should be tested
Current testing guidelines focus on people who are at higher risk of being infected with TB bacteria. The CDC recommends that the following people be tested:16
People who could likely be exposed to TB disease:
- People who have spent time with someone who has TB disease
- People from a country where TB disease is common which include: most countries in Latin America, the Caribbean, Africa, Asia, Eastern Europe, and Russia
- People who live or work in high-risk settings such as long-term care facilities or nursing homes, homeless shelters, or prisons
- Healthcare workers who care for patients at increased risk for TB disease
- Infants, children, and adolescents exposed to adults who are at increased risk for LTBI or TB disease
- People who are likely to develop TB disease if they have LTBI:17
- People with HIV infection
- People who became infected with TB bacteria in the last two years
- Babies and young children
- People who inject illegal drugs
- People who are sick with other diseases that weaken the immune system.
- Elderly people
- People who were not treated correctly for TB in the past
- People who are receiving immunosuppressive therapy (TNF-alpha antagonists, corticosteroids ≥15 mg/day of prednisone, or immunosuppressive drug therapy following organ transplantation
- People with silicosis; chronic renal failure; leukemia; or cancer of the head, neck, or lung
- People with diabetes mellitus
Labs can be powerful players in the drive to end TB
Despite strong progress to eradicate TB around the world, it remains a serious infectious disease that has plagued humanity continuously throughout history. People today are more globally mobile than ever, and we take our health status with us, as the world was reminded recently with COVID-19.
Powerful modern diagnostics give us a significant advantage to find and treat TB before people become sick or contagious. As increasingly important partners in the healthcare delivery system, medical laboratories can bring precision and expertise to make diagnosing LTBI faster, easier, and less subjective than in the past, making now the time to end TB for good.
REFERENCES
1. Tuberculosis. Who.int. Accessed March 3, 2023. https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
2. Tucker A. The great New England vampire panic. Smithsonian Magazine. Published September 30, 2012. Accessed March 3, 2023. https://www.smithsonianmag.com/history/the-great-new-england-vampire-panic-36482878/.
3. CDCTB. Data and Statistics. Centers for Disease Control and Prevention. Published November 29, 2022. Accessed March 3, 2023. https://www.cdc.gov/tb/statistics/default.htm.
4. Tuberculosis. Who.int. Accessed March 3, 2023. https://www.who.int/health-topics/tuberculosis.
5. CDCTB. Basic TB facts. Centers for Disease Control and Prevention. Published July 25, 2022. Accessed March 3, 2023. https://www.cdc.gov/tb/topic/basics/default.htm.
6. Drain PK, Bajema KL, Dowdy D, et al. Incipient and subclinical tuberculosis: A clinical review of early stages and progression of infection. Clin Microbiol Rev. 2018;31(4). doi:10.1128/CMR.00021-18.
7. CDCTB. Treatment regimens for latent TB infection. Centers for Disease Control and Prevention. Published September 1, 2022. Accessed March 3, 2023. https://www.cdc.gov/tb/topic/treatment/ltbi.htm.
8. CDCTB. Treatment for TB disease. Centers for Disease Control and Prevention. Published July 26, 2022. Accessed March 3, 2023. https://www.cdc.gov/tb/topic/treatment/tbdisease.htm.
9. Cdc.gov. Accessed March 3, 2023. https://www.cdc.gov/globalhivtb/images/DGHT-TB-Factsheet.pdf.
10. The end TB strategy. Who.int. Accessed March 3, 2023. https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy.
11. Latent TB infection testing and treatment: Summary of U.S. recommendations. Cdc.gov. Published 2020. Accessed March 3, 2023. https://www.cdc.gov/tb/publications/ltbi/pdf/CDC-USPSTF-LTBI-Testing-Treatment-Recommendations-508.pdf.
12. Barcellini L, Borroni E, Brown J, Brunetti E, et al. First independent evaluation of QuantiFERON-TB Plus performance. Eur Respir J. 2016;47(5):1587-90. doi:10.1183/13993003.02033-2015.
13. Barcellini L, Borroni E, Brown J, Brunetti E, et al. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Respir J. 2016;48(5):1411-1419. doi:10.1183/13993003.00510-2016.
14. Viana Machado F, Morais C, Santos S, Reis R. Evaluation of CD8+ response in QuantiFERON-TB Gold Plus as a marker of recent infection. Respir Med. 2021;185:106508. doi:10.1016/j.rmed.2021.106508.
15. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):111-115. doi:10.1093/cid/ciw778.
16. CDCTB. Who should be tested for TB infection. Centers for Disease Control and Prevention. Published August 30, 2022. Accessed March 3, 2023. https://www.cdc.gov/tb/topic/testing/whobetested.htm.
17. Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020;69(1):1-11. doi:10.15585/mmwr.rr6901a1.
About the Author

Parth Patel, DMSc, PA-C
is a board-certified Physician Associate with a terminal degree of Doctor of Medical Science. He is a Medical Science Liaison supporting the Immune Response in North America for QIAGEN including, QuantiFERON-Gold TB Plus. Prior to joining QIAGEN, he specialized in the field of medical dermatology and routinely screened patients for LTBI prior to initiation of immunosuppressive therapy.