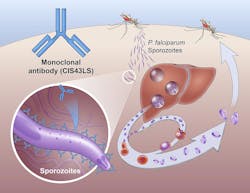
Monoclonal antibody prevents malaria infection in African adults
One dose of an antibody drug safely protected healthy, non-pregnant adults from malaria infection during an intense six-month malaria season in Mali, Africa, a National Institutes of Health clinical trial has found. The antibody was up to 88.2% effective at preventing infection over a 24-week period, demonstrating for the first time that a monoclonal antibody can prevent malaria infection in an endemic region. These findings were published in The New England Journal of Medicine and presented at the American Society of Tropical Medicine & Hygiene 2022 Annual Meeting in Seattle.
The Phase 2 NIAID-USTTB trial evaluated the safety and efficacy of a one-time, intravenous infusion of a monoclonal antibody called CIS43LS. This antibody was previously shown to neutralize the sporozoites of P. falciparum in the skin and blood before they could infect liver cells. Researchers led by Robert A. Seder, M.D., isolated a naturally occurring form of this antibody from the blood of a volunteer who had received an investigational malaria vaccine, and then modified the antibody to extend the length of time it would remain in the bloodstream. Dr. Seder is the acting chief medical officer and acting associate director of the NIAID Vaccine Research Center (VRC) and chief of the VRC’s Cellular Immunology Section.
The study team for the Phase 2 trial enrolled 369 healthy, non-pregnant adults aged 18 to 55 years in the rural communities of Kalifabougou and Torodo in Mali, where intense P. falciparum transmission typically occurs from July through December each year.
The first part of the trial assessed the safety of three different doses of CIS43LS — 5 milligrams per kilogram of body weight, 10 mg/kg and 40 mg/kg — administered by intravenous infusion in 18 study participants, with six participants per dose level. The study team followed these participants for 24 weeks and found the antibody infusions were safe and well-tolerated.
The second part of the trial assessed the efficacy of two different doses of CIS43LS compared to a placebo. Three hundred and thirty participants were assigned at random to receive either 10 mg/kg of the antibody, 40 mg/kg, or a placebo by intravenous infusion. No one knew who was assigned to which group until the end of the trial. The study team followed these individuals for 24 weeks, testing their blood for P. falciparum weekly for the first 28 days and every two weeks thereafter. Any participant who developed symptomatic malaria during the trial received standard treatment from the study team.
The investigators analyzed the efficacy of CIS43LS two ways. Based on the time to first P. falciparum infection over the 24-week study period, the high dose (40 mg/kg) of CIS43LS was 88.2% effective at preventing infection and the lower dose (10 mg/kg) was 75% effective. An analysis of the proportion of participants infected with P. falciparum at any time over the 24-week study period found the high dose was 76.7% at preventing infection and the lower dose was 54.2% effective.