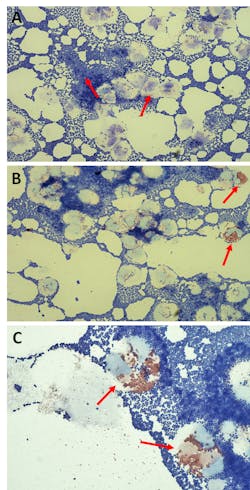
Cerebrospinal fluid findings in neonatal Group B streptococcal meningitis
Preserving cellular integrity in cerebrospinal fluid (CSF) specimens can be challenging in laboratory practice. Delays in transporting samples or in processing them can cause cellular degeneration. However, cellular degeneration also can occur on rare occasions even when the specimens arrive at the lab and are processed within acceptable time parameters.
Just such a case occurred with specimens for an infant diagnosed with severe late-onset Group B streptococcal meningitis at Stony Brook University Hospital in New York. Due to widespread cellular degeneration, an accurate CSF cell differential could not be performed. GBS grew in peripheral blood and CSF cultures, confirming the diagnosis.
A potential explanation for this unusual rapid form of WBC degeneration is a recent finding that certain bacterial pathogens can accelerate the process of cell degeneration.
At 27-days-old, the infant arrived in the emergency department with fever, nasal congestion, shortness of breath, and decreased responsiveness. Her rectal temperature and heart rate were 38.7 C and 233 bpm, respectively. Cerebrospinal fluid (CSF), urine, and peripheral blood analyses were obtained, and intravenous therapy with ampicillin, ceftazidime, and acyclovir was initiated.
The infant described in this case was born at 36 weeks gestation (preterm) to a mother who had negative prenatal labs, including Group B streptococcus (GBS). The mother’s pregnancy was uncomplicated, including her delivery and postnatal period.
Early- vs late-onset GBS disease
GBS is a primary cause of neonatal septicemia and meningitis, and it is categorized into early-onset and late-onset diseases (EOD and LOD).1,2 Neonatal Group B streptococcal meningitis is not always attributable to the mother and can occur in infants, even when maternal GBS screens are negative.
EOD affects infants ages 1 to 6 days old via vertical transmission, before or during delivery, when GBS is present in the mothers’ genital tracts and anorectal sites.1,2 Many of the infants developed bacteremia and pneumonia,3 with low incidence of meningitis.3,4
False negative prenatal GBS culture screens contributed to most cases of EOD, primarily due to suboptimal specimens for culture or to maternal conversion from GBS negative to positive after preliminary culture.5 Although time-consuming with a 36-48-hour incubation period, a vaginal-rectal culture continues to be a gold standard for routine GBS screen, owing to its 98.4% sensitivity, compared to >90% sensitivity for polymerase chain reaction (PCR) with a 1-4-hour turnaround time.5
In contrast, LOD occurs in infants between 7 and 89 days of life.2,6 Multiple potential modes of transmission include: horizontal (spread of infection from one infant to another through shared healthcare givers),7 vertical (by breastfeeding infected milk),8 nosocomial (e.g., use of contaminated medical equipment),7 or genetically driven (i.e., GBS as normal intestinal flora of neonates likely developed virulence factors, which facilitated it to cross intestinal mucosa and blood-brain barrier).9 LOD is usually associated with bacteremia and meningitis,3,5 and neurological sequelae are common in severe cases.2
Prevention measures, such as a routine prenatal GBS screen and intrapartum antibiotic prophylaxis (IAP), have significantly decreased the incidence rate of EOD from 1.7 to 0.4 per 1000 live births since 1990; however, LOD did not benefit from these interventions, and the incidence rate remains 0.3 to 0.4 per 1000 live births.5 Availability of an effective vaccine against GBS is a promising tool that would eventually lower the incidence rate of both LOD and EOD.6
Clinical and laboratory findings in GBS meningitis
In the case described here, the white blood cell (WBC) count in peripheral blood was 1.48 x 103/µL (7.0-17.0 x 103/µL), with a differential of 14% polymorphonuclear neutrophils (PMNs), 7% band neutrophils, 67% lymphocytes, 2% atypical lymphocytes, 6% monocytes, 1% basophils, and 3% metamyelocytes. CSF was turbid, with a protein of 196.5 mg/dL (20.0-120.0 mg/dL), glucose of <2.0 mg/dL (40.0-70.0 mg/dL), WBC count of 159/µL (0-30/µL). In addition, a bacterial antigen was positive for GBS. Many Gram-positive cocci (GPC), moderate degenerated white blood cells (WBCs), and rare to few eosinophils were observed on CSF Wright-Giemsa stain (Figure 1).
The infant was diagnosed with severe late-onset Group B streptococcal meningitis, following observation of seizures, detection of tiny abscess and leptomeningeal enhancement consistent with meningitis by magnetic resonance imaging (MRI) of the brain. She was treated with ampicillin and gentamicin, until blood and CSF cultures cleared, then completed a 4-week course of therapy with penicillin.
Her condition had clinically improved after 15 days of critical care, and she completed the course of therapy at home. She was also discharged on phenobarbital, with continued monitoring for GBS-related sequelae.
CSF eosinophilia in GBS meningitis
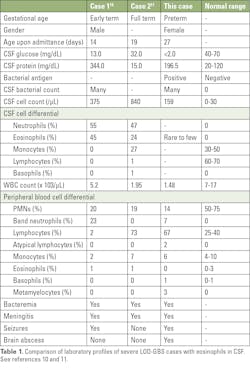
The occurrence of eosinophils in CSF of neonates with severe LOD-GBS is rare. We found only two cases reported in the medical literature prior to the case at Stony Brook.10,11 (Table 1). All three cases exhibited CSF pleocytosis, hypoglycorrhachia, bacteremia, peripheral blood leukopenia, and neutropenia.
In the past, the significance of eosinophils focused on their involvement in parasitic infections and allergic reactions, but current knowledge of their functions suggests that they can be activated by bacteria,12 engaging in bacterial killing and phagocytosis in inflammatory sites.13
Thus, the presence of eosinophils in the CSF of the infant at Stony Brook and the two earlier cases was probably induced by GBS, and their recruitment was most likely facilitated by interleukin-5 (IL-5) and eotaxin-1. IL-5 is a cytokine that regulates eosinophil growth, activation, survival, and migration; eotaxin-1 is a chemokine that stimulates chemotaxis in eosinophils.14
Moreover, the occurrence of basophils in Case 2 (Table 1) was possibly related to their recently recognized functions as antigen-presenting cells and as regulators of adaptive immunity.15 Overwhelming sepsis and decreased bone marrow production have potentially caused the reduction of WBCs and neutrophils, as well as the emergence of immature cells in peripheral blood.Significance of CSF cellular degeneration
Cellular degeneration is a descriptive term for disintegrated, or lysed, cells observed on body fluid stains (e.g. Gram stain, Wright-Giemsa stain). Because degenerated cells lack intact cytoplasm, and cytoplasmic contents are often missing or morphologically distorted, they are difficult to distinguish and are excluded from differential (Figure 1). In cases where cells are excessively degenerated, a differential is not reported, potentially affecting clinical decisions in situations where there is a need to differentiate between bacterial and aseptic meningitis. Sometimes, clinicians may request that the laboratory identify the WBCs and estimate their numbers if possible.
CSF cellular degeneration is known to be caused by delayed transportation and processing time. Removal of CSF from the body may lead to lysis of WBCs as a result of increased pH,16 hypotonicity, and decreased concentrations of membrane-stabilizing proteins and lipids.17
Neutrophils in CSF have a very short life span in vitro (7-h viability). They lyse faster than monocytes and lymphocytes and show a reduction rate of 32% after 1 hour and 50% after 2 hours at room temperature.18 The present case had a specimen transportation and reporting time of 0.18 h (< 1.0 h) and 0.89 h (< 1.15 h), respectively; therefore, processing delays do not readily explain the approximately 90% degenerated WBCs present on the CSF Wright-Giemsa stain of the patient.
Similarly, Stony Brook had a 12-year-old male patient who had a neurosurgery for hydrocephalus that was complicated by Streptococcus mitis/oralis group. There was no delay in specimen transportation and reporting time (0.42 h and 1.10 h, respectively), but the CSF Wright-Giemsa stain showed many GPC and many degenerated WBCs. CSF cell differential was unavailable, as WBCs were too degenerated.
A potential explanation for this unusual rapid form of WBC degeneration is a recent finding that certain bacterial pathogens (e.g. Streptococcus, Staphylococcus, Listeria, Borrelia, Burkholderia) can accelerate apoptosis or induce PMN lysis to escape intracellular killing via activation of pro-death Bcl-2 protein family.19 We hypothesize that the large numbers of streptococci seen on the CSF Wright-Giemsa stains in both of these cases triggered extensive apoptosis, leading to the cellular degeneration seen on the slides. While several methods have been proposed for preserving CSF cellular integrity when processing is delayed (e.g. addition of serum-containing medium or Earle’s balanced solution with human serum albumin to CSF specimen, spiking of CSF cells into 5% fetal calf serum or saline, refrigeration of CSF specimen, minimum centrifugation procedure19), these steps are difficult to implement in routine clinical practice and may not help for CSF specimens with moderate to many bacterial pathogens that are known to trigger PMN lysis or accelerate apoptosis.
In conclusion, preserving cellular integrity in cerebrospinal fluid (CSF) specimens can be challenging in laboratory practice. In this case, neutrophils in the CSF showed cellular degeneration despite short time to analysis, and occasional eosinophils were present. Neutrophil degeneration in the setting of overwhelming bacteria may be due to accelerated apoptosis. In these cases, it may be appropriate for the lab to provide a descriptive or qualitative report of the WBC morphology for this type of slide. A practical approach to this situation is to refer the CSF stain to a pathologist or lead technologist, because they may be able to classify the degenerated WBCs based on their morphological features, which would help with the clinical interpretation.
Acknowledgements
The authors wish to thank Lorillaine Villasis, MPH; Rebecca Riemann, MT; Bruce Kube, MS; Aderonke Adefisayo, MD; and Eric D. Spitzer, MD, PhD, for their expert technical assistance, clinical input, and review of the manuscript.
References:
- Melin P. Neonatal group B streptococcal disease: from pathogenesis to preventive strategies. Clin Microbiol Infec. 2011;17(9):1294-1303. doi: 10.1111/j.1469-0691.2011.03576.x.
- Berardi A, Rossi C, Lugli L, et al. Group B streptococcus late-onset disease: 2003-2010. Pediatrics. 2013;131(2):e361-e368. DOI: 10.1542/peds.2012-123.
- Phares C, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008;299(17):2056-2065. doi: 10.1001/jama.299.17.2056.
- Poyart C, Reglier-Poupet H, Tazi A, et al. Invasive group B streptococcal infections in infants, France. Emerg Infect Dis. 2008;14(10):1647-1649. doi: 10.3201/eid1410.080185.
- Ahmadzia HK, Heine RP. Diagnosis and management of group B streptococcus in pregnancy. Obstet Gynecol Clin N Am. 2014;41(4):629-647. doi: 10.1016/j.ogc.2014.08.009.
- Dudek C, Shah C, Zayas J, et al. The many faces of late onset group B streptococcus infection. Pediatr Infect Dis J. 2016;1(3):14. http://pediatric-infectious-disease.imedpub.com.
- Morinis J, Shah J, Murthy P, et al. Horizontal transmission of group B streptococcus in a neonatal intensive care unit. Pedriatr Child Health. 2011;16(6):e48-e50. doi: 10.1093/pch/16.6.e48.
- Olver WJ, Bond DW, Boswell TC, et al. Neonatal group B streptococcal disease associated with infected breast milk. Arch Dis Child Fetal Neonatal Ed. 2000;83:F48-F49. doi: 10.1136/fn.83.1.f48.
- Libster R, Edwards KM, Levent F, et al. Long-term outcomes of group B streptococcal meningitis. Pediatrics. 2012;130(1):e8-e15. doi: 10.1542/peds.2011-3453.
- Miron D, Snelling LK, Josephson SL, et al. Eosinophilic meningitis in a newborn with group B streptococcal infection. Pediatr Infect Dis J. 1993;12(11):966-967. doi: 10.1097/00006454-199311000-00021.
- Hewett FA, Hendley JO. Eosinophilic meningitis in a neonate. Clin Pediatr. 2002;41(4):269-271. doi: 10.1177/000992280204100412.
- Svensson L, Wenneras C. Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect. 2005;7(4):720-728.
- Hogan SP, Waddell A, Fulkerson PC. Eosinophils in infection and intestinal immunity. Curr Opin Gastroenterol. 2013;29(1):7-14. doi: 10.1016/j.micinf.2005.01.010.
- Collins PD, Marleau S, Griffiths-Johnson DA, et al. 1995. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182(4):1169-1174. doi: 10.1084/jem.182.4.1169.
- Sokol CL, Medzhitov R. Emerging functions of basophils in protective and allergic immune responses. Mucosal Immunol. 2010;3(2):129-137. doi: 10.1038/mi.2009.137.
- Cunniffe JG, Whitby-Strevens S, Wilcox MH. Effect of pH changes in cerebrospinal fluid specimens on bacterial survival and antigen test results. J Clin Pathol. 1996;49(3):249-253. DOI: 10.1136/jcp.49.3.249.
- Steele RW, Marmer DJ, O’Brien MD, et al. Leukocyte survival in cerebrospinal fluid. J Clin Microbiol. 1986;23(5):965-966. doi: 10.1128/JCM.23.5.965-966.1986.
- McCracken JM, Allen LA. Regulation of human neutrophil apoptosis and lifespan in health and disease. J Cell Death. 2014;7:15-23. doi: 10.4137/JCD.S11038.
- de Graaf MT, van den Broek PD, Kraan J, et al. Addition of serum-containing medium to cerebrospinal fluid prevents cellular loss over time. J. Neurol. 2011;258(8):1507-1512. doi: 10.1007/s00415-011-5970-8.
About the Author

Marianne H. Travis, MLS-ASCP, MS
is a Medical Laboratory Scientist at Stony Brook University Medical Center. She is a Laboratory Technologist in the Clinical Microbiology section of the Department of Pathology at Stony Brook University Medical Center.

Christy Beneri, DO
is Associate Professor of Pediatrics at Stony Brook University. She serves as Program Director for Pediatric Infectious Diseases at Stony Brook Children’s Hospital.

Lisa Senzel, MD, PhD
is a Clinical Associate Professor of Pathology at Stony Brook University. She serves as Chief of the Core Laboratory and Associate Chief of Transfusion Services at Stony Brook University Medical Center.