As the first wave of the novel coronavirus (2019-nCoV) arrived on the shores of Washington state, the need for rapid infection detection arrived as well. Since that time, governmental agencies such as the CDC, FDA and WHO stressed the importance of having accurate and fast diagnostic test options that could meet growing concerns and growing cases of what has become known as COVID-19.
With this in mind, many diagnostics companies shifted some in-house priorities and dedicated personnel to creating RT-PCR and/or antibody tests that could offer some relief to increased patients and demands for test results that could confirm or negate suspected infections.
While many diagnostics companies worked hard to be the first to cross the industry finish line with an FDA-approved test for nationwide distribution, additional companies worked just as hard to create the consumables and disposables required of some new tests.
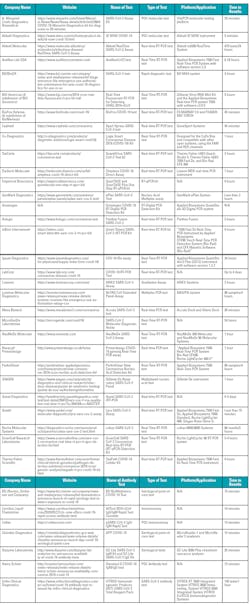
The table below lists the tests that have garnered FDA approval as of April 8, with antibody tests listed separately from PCR tests. With a number of dedicated companies still working on creating first or additional tests, or who are waiting on FDA approval of a previously submitted test, we can be sure there will be more waves of test options to follow.