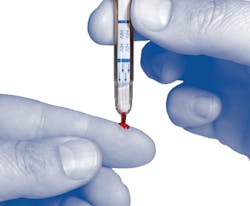
Point-of-Care HIV-1/2 Assay
The SURE CHECK rapid POC test detects antibodies to HIV-1 and HIV-2 in fingerstick whole blood, venous whole blood, serum and plasma specimens. Key features include a required sample size of only 2.5 µl of fingerstick blood, sensitivity of 99.7 percent and specificity of 99.9 percent.
Universal Transport Medium
The Universal Transport Medium is intended for the collection and preservation of viruses, chlamydia, mycoplasma and ureaplasma specimens from the collection site to the testing lab. Kits include a vial and individually wrapped sterile PurFlock Ultra or polyester tipped swabs. Available in 1ml or 3ml fill, the medium is stable at room temperature.
Infectious Disease Menu
The comprehensive Atellica IM infectious disease menu includes HBsAg II. Combined with engineering innovations and detection technology, it provides the high sensitivity and precision that is critical for accurate detection of viral hepatitis or HIV.
HIV-1 Genotyping Assay
The FDA-approved Sentosa SQ HIV-1 genotyping assay combines detection of HIV-1 Group M drug resistance mutations (DRMs) in the protease, reverse transcriptase and integrase regions of the pol gene, all in a single test. The assay runs on an automated NGS workflow, which allows for higher sensitivity of mutation detection.
HPV Molecular Program
The WSLH Proficiency Testing’s new HPV molecular program includes five samples, shipped three times a year. The 1mL samples are compatible with Roche COBAS, Cepheid GeneXpert and Hologic Aptima platforms and more. HPV qualitative and genotyping reporting is available.
About the Author
Sign up for our eNewsletters
Get the latest news and updates