The role of race and ethnicity in variant hemoglobin traits
The United States is a culturally diverse nation and a melting pot comprised of a variety of ethnicities and races. Race and ethnicity play an important role in classifying diabetes – a commonly occurring disease in the United States. In 2018, 34.1 million adults aged 18 years or older – or 13.0 percent of all U.S. adults – had diabetes. In addition, 7.3 million adults aged 18 years or older who met laboratory criteria for diabetes were not aware of or did not report having diabetes.
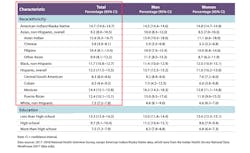
The prevalence of diagnosed diabetes was highest among American Indians/Alaska natives (14.7 percent), followed by people of Hispanic origin (12.5 percent), non-Hispanic blacks (11.7 percent), non-Hispanic Asians (9.2 percent) and non-Hispanic whites (7.5 percent). 1 (See Table 1)
This article will examine the role of A1c in diabetics from diverse racial and ethnic backgrounds. It also will describe the importance of detecting a variant – a situation that is of particular relevance in ethnic and racial populations where these variants are more common.
A1c testing in variant hemoglobin carriers
Hemoglobin A1c is an important analyte for the detection and diagnosis of diabetes. It is a measure of the amount of glucose attached to hemoglobin (Hb) in red blood cells over the typical 120-day lifespan of a normal red blood cell. The A1c test reflects the average blood glucose over the preceding 2-3 months. Therefore, it is also an effective long-term glycemic monitor for diabetes management. There are many advantages of using HbA1c as a glycemic marker: It does not require fasting and can be taken 2-4 times a month, depending upon the diabetic condition of the individual. It also is a great monitoring analyte and can help prevent diabetes-related complications.
But just as is the case with any other protein entity in the body, hemoglobins are susceptible to mutations. Hemoglobin by definition is Heme + globin. Heme means iron and globin is a protein made of amino acid chains. When a mutation occurs in a single amino acid of the globin protein, it results in a variant. These variants can be homozygous or heterozygous. For example, an individual with a mutation in the 6th position of the globin protein manifests the condition as an HbS variant. If the individual inherits a copy of the HbS variant from each parent, the disease manifests itself as sickle cell disease. If the individual inherits one copy of the variant gene from one of the parents, he is said to be a carrier and is usually asymptomatic.
Any charge-based separation method of HbA1c testing has the ability to detect commonly occurring variant hemoglobins, such as S, C, D and E, and has the added advantage of calling to attention the presence of the variant in the form of a hemoglobin profile with components of the hemoglobin elucidated as peaks in the order that they get separated.
Incidence of variant hemoglobins in ethnic populations
Hemoglobin variants are more common than one would think. A comprehensive table in the National Institute of Diabetes and Digestive and Kidney disease (NIDDK) explains the incidence of the most commonly occurring hemoglobins in the various ethnic populations and races. Here is a summary of the information in that table.2
HbS is a variant that is found in African Americans and Hispanic Americans/Latinos. It is also found in Africa, South or Central America (especially Panama), the Caribbean islands, Mediterranean countries (such as Turkey, Greece, and Italy), India, and Saudi Arabia.
When an individual carries two abnormal HbS genes, the individual is homozygous for HbS. This individual has Sickle cell anemia (also called HbSS disease) where the sickled red blood cells interfere with circulation and decreased life span of red blood cells. This can result in hemolytic, splenic sequestration, aplastic crises and multiple complications. Sickle cell anemia occurs in 1 of every 500 African American births and in 1 of every 36,000 Hispanic/Latino births.3
When an individual carries one normal gene HbA and one abnormal gene HbS, the individual is heterozygous for HbS, or is said to carry the HbS trait. These individuals are generally asymptomatic. In the United States, about 1 out of 12 African Americans and about 1 out of 100 Hispanic Americans/Latinos have a sickle cell trait.2 It is noteworthy that 11.7 percent of the African American diabetic population can also potentially be carriers of the HbS variant hemoglobin trait at a frequency of 1 out of 12 individuals. Similarly, 12.5 percent of the Hispanic American/Latino population has diabetes, and 1 out of 12 individuals of this population can be carrying the asymptomatic HbS trait.
HbC is a variant that is found in African Americans and people of West African descent. About 2.3 percent of African Americans have the HbC trait. The HbC trait (also called HbAC) is asymptomatic. Individuals with HbC disease (also called HbCC disease) exhibit mild hemolytic anemia and mild to moderate enlargement of the spleen.4
HbE is a variant that is found in Asian Americans, especially those of Southeast Asian descent. It is common in Cambodia, Indonesia, Laos, Malaysia, Thailand, and Vietnam and is also seen in southern China, India, the Philippines, and Turkey. Prevalence of HbE may be 30 percent in Southeast Asia. Individuals with HbE disease (also called HbEE disease) exhibit mild hemolytic anemia, microcytosis, and mild enlargement of the spleen.4
In addition to these commonly occurring hemoglobins, there are thousands of other variant hemoglobins that have been documented and many that are yet to be discovered.5
Geographical categorization of ethnic populations in the United States
How can knowledge about the prevalence of diabetes in ethnic populations combined with the prevalence of variant traits in some of these same population groups be applied to the U.S. population? And how should laboratorians and physicians apply this information to their decisions about which A1c testing method to choose?
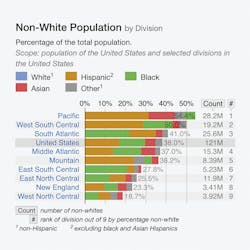
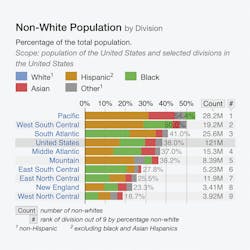
The effect of population migration from parts of the world where variants HbS, C, D and E variants are common to various regions in the United States can be visualized in Table 2.
Table 2 shows the categorization of Hispanics, Blacks, Asians and others in selected divisions in the United States.6
The United States Census Bureau defines four statistical regions, with nine divisions:7
- Region 1: Northeast
Division 1: New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont)
Division 2: Mid-Atlantic (New Jersey, New York, and Pennsylvania)
- Region 2: Midwest
Division 3: East North Central (Illinois, Indiana, Michigan, Ohio, and Wisconsin)
Division 4: West North Central (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota)
- Region 3: South
Division 5: South Atlantic (Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, District of Columbia, and West Virginia)
Division 6: East South Central (Alabama, Kentucky, Mississippi, and Tennessee)
Division 7: West South Central (Arkansas, Louisiana, Oklahoma, and Texas)
- Region 4: West
Division 8: Mountain (Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, and Wyoming)
Division 9: Pacific (Alaska, California, Hawaii, Oregon, and Washington)
For example, the graph shows that the Pacific Division has the maximum number of Hispanics and the South Atlantic and East South Central Divisions have the maximum number of Blacks. HbS and HbC is commonly found in these two populations. The graph also identifies the pockets of Asians, and the possibility of HbD and HbE variants in these populations cannot be ruled out.
A1c testing methods that detect variants traits
Not all A1c testing methods are the same. Charge-based separation methods, such as ion exchange HPLC and Capillary electrophoresis, are able to detect variant hemoglobins, and structure-based separation methods, such as immunoassays and boronate affinity chromatography, do not have the ability to detect variants. Charge-based separation methods provide a hemoglobin peak separation profile in the form of a chromatogram. The method is designed to quantitate A1c but also presumptively identify the most commonly occurring variant hemoglobins. Immunoassays rely on the specificity of an antibody targeted to the N-terminal glycated amino acids on the ß chain to quantify A1c. The A1c result is a number with no additional variant information. There is no way to know if there is a variant hemoglobin or not.
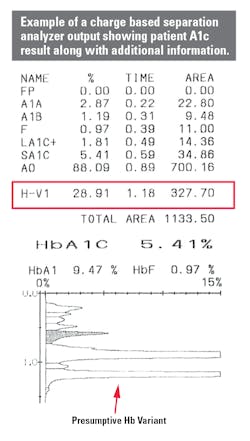
Figure 1 is an example of an A1c result using a method that shows the entire profile of hemoglobin separation. The chromatography clearly shows the separation of peaks. The result table calls out the presence of a variant (H-VAR) based on the retention time around which the peak elutes.
Summary
Each region is unique with its pockets of ethnic diversity. It is beneficial for laboratorians and physicians to adopt an A1c testing method that takes into consideration the testing samples that arrive at the laboratory from various pockets of human diversity. There are some ethnic and racial populations where the prevalence of diabetes is greater than in other populations. Combining that knowledge with information on the occurrence of the most commonly occurring variants and the spread of various ethnic groups across the nation, gives decision-makers a fair idea of which A1c testing method would be the best fit for their geographical area.
References:
- National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 2, 2020.
- Sickle Cell Trait & Other Hemoglobinopathies & Diabetes (For Providers). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/sickle-cell-trait-hemoglobinopathies-diabetes. Accessed December 2, 2020.
- Sickle Cell Disease. National Heart, Lung, and Blood Institute. www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_WhatIs.html. Accessed on December 2, 2020.
- Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001; 47(2): 153–163.
- Thom C, Dickson C, Gell D, Weiss M. Hemoglobin variants: Biochemical properties and clinical correlates. Cold Spring Harbor Perspective Medicine. 2013; 3(3): a011858. https://statisticalatlas.com/United-States/Race-and-Ethnicity. Accessed December 2, 2020.
- Census Regions and Divisions in the United States. United States Census Bureau. Geography Division. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf. Accessed December 2, 2020.
About the Author

Priya Sivaraman, PhD
is a Senior Product Manager at Tosoh Bioscience Inc. based in Grove City, Ohio. She specializes in HbA1c testing. With more than 15 years of experience in the diagnostic industry, she supports a broad profile of A1c clients in the hospital and laboratory setting.