Evolving paradigms in diabetes diagnosis and classification: ADA standards of care 2025 and the use of artificial intelligence
To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
1. Describe the pathophysiologies in the classifications of diabetes.
2. Discuss the laboratory tests and their results in the diagnosis of the classifications of diabetes.
3. Differentiate confirmatory data of laboratory testing in the confirmation of diabetes.
4. Discuss AI strategies for the prediction and management of diabetes.
Diabetes mellitus is a chronic, metabolic disease that occurs due to the body’s inability to produce enough insulin or to ineffectively utilize the insulin produced leading to hyperglycemia or elevated levels of blood glucose (or blood sugar). If not controlled, diabetes can cause damage to the heart, blood vessels, eyes, kidneys, and nerves.1
Per the International Diabetes Federation’s Diabetes Atlas 11th edition published in 2025, diabetes is one of the fastest growing global health emergencies in the 21st century with 1 in 9 adults having diabetes. In 2024, 588.7 million people had diabetes, and it is estimated that 852.5 million would develop diabetes by 2050 with a 45% growth. In the United States, 65.6 million people have diabetes, and it is estimated that number to reach 72.4 million by 2050 with a 10% rise.1
The field of diabetes care is evolving through new research, technology, and treatments to improve the health and well-being of people with diabetes. Since 1989, the American Diabetes Association (ADA) has been updating the Standards of Care recommendations annually to capture the most current state in the field of diabetes.
Classification of diabetes
The America Diabetes Association (ADA) classified diabetes based on metabolic, genetic, and pathophysiology features, as below:2
- Type 1 diabetes (T1D) – due to autoimmune β-cell destruction, usually leading to total insulin deficiency, including latent autoimmune diabetes in adults
- Type 2 diabetes (T2D) – due to a non-autoimmune progressive loss of adequate β-cell insulin secretion, frequently causing progressive insulin resistance
- Specific types of diabetes due to other causes, e.g.,
- monogenic diabetes caused from a mutation in a single gene
- diseases of the exocrine pancreas
- drug- or chemical-induced diabetes - Gestational diabetes mellitus (GDM) – observed in the second or third trimester of pregnancy in some individuals
It is important to identify the type of diabetes — type 1 or type 2 — to render personalized therapy. Traditionally it is believed that type 1 occurs in children and type 2 in adults; however, that may not be the case always. For some individuals, it is difficult to clearly classify the diabetes type at the time of diagnosis, and misdiagnosis is common. About 40% of type 1 diabetes cases are misdiagnosed as type 2 and many maturity-onset diabetes of the young (MODY) caused by monogenic syndrome may be misdiagnosed as type 1 diabetes.3
AABBCC is a clinical tool that may be used to determine if a newly diagnosed diabetic has type 1 diabetes based on the following criteria:
A) Age (e.g., for individuals <35 years old, consider type 1 diabetes)
A) Autoimmunity (e.g., personal or family history of autoimmune disease or polyglandular autoimmune syndromes)
B) Body habitus (e.g., BMI <25 kg/m2)
B) Background (e.g., family history of type 1 diabetes)
C) Control (preferred term is “goal,” i.e., the inability to achieve glycemic goals on noninsulin therapies)
C) Comorbidities (e.g., treatment with immune checkpoint inhibitors for cancer can cause acute autoimmune type 1 diabetes)
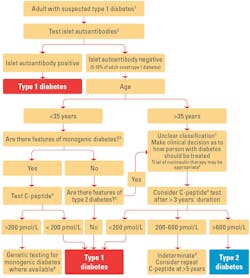
The American Diabetes Association encourages use of C-peptide and islet autoantibody testing in ambiguous adult-onset cases. The flowchart in Figure 1 helps to distinguish type 1 and type 2 diabetes using age, BMI, autoantibodies, and insulin dependency.
Tests for screening and diagnosis
- Fasting plasma glucose (FPG)
- 2-h plasma glucose (2-h PG) during a 75-g oral glucose tolerance test (OGTT)
- A1C
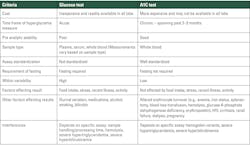
The A1C test should be performed using a method that is certified by the National Glycohemoglobin Standardization Program (NGSP) (ngsp.org) and standardized or traceable to the Diabetes Control and Complications Trial (DCCT) reference assay. Point-of-care A1C assays may be NGSP certified and cleared by the U.S. Food and Drug Administration (FDA) for use in both Clinical Laboratory Improvement Amendments (CLIA)–regulated and CLIA-waived settings.2
Confirming the diagnosis
Unless there is an absolute match between the clinical diagnosis (e.g., individual with hyperglycemia or hyperglycemic crisis and random plasma glucose >200 mg/dL (>11.1 mmol/L), additional confirmatory tests are necessary. Diabetes can be confirmed with two abnormal screening test results measured either at the same time or at two different points of time and may be performed using two different types of tests, e.g., A1C and FPG.2
Criteria for diabetes in non-pregnant individuals includes one of the following:
- A1C ≥6.5% (>48 mmol/mol)
- Fasting plasma glucose (FPG) ≥126 mg/dL (≥7.0 mmol/L)
- 2-hour plasma glucose (2-h PG) ≥200 mg/dL (≥11.1 mmol/L) during OGTT
- Random plasma glucose ≥200 mg/dL in symptomatic patients
Criteria for pre-diabetes in non-pregnant individuals includes one of the following:
- A1C 5.7–6.4% (39–47 mmol/mol)
- Fasting plasma glucose (FPG) 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L)
- 2-hour plasma glucose (2-h PG) ≥200 mg/dL (≥11.1 mmol/L) during 2-h PG during 75-g OGTT 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L) impaired glucose tolerance (IGT)
Type 1 diabetes
5–10% of diabetics have type 1 diabetes.2 For individuals with a family history of type 1 diabetes or other genetic risks, screening for presymptomatic type 1 diabetes (T1D) may be done by using standardized islet autoantibody test for detection of autoantibodies to insulin, glutamic acid decarboxylase (GAD), islet antigen 2 (IA-2), or zinc transporter 8 (ZnT8). Multiple confirmed islet autoantibodies are a risk factor for clinical diabetes. An individual may be in different stages of type 1 diabetes as depicted in Table 2.
Prediabetes and type 2 diabetes
90–95% of diabetics have type 2 diabetes.2 Criteria to screen for pre-diabetes or type 2 diabetes is as follows:
- Adults who are overweight or obese (BMI 25 kg/m2 or 23 kg/m2 in individuals of Asian ancestry) and have one or more of the following risk factors:
- First-degree relative with diabetes
- High-risk race, ethnicity, and ancestry (i.e., African American, Latino, Native American, Asian American)
- History of cardiovascular disease
- Hypertension (130/80 mmHg or on therapy for hypertension)
- HDL cholesterol level <35 mg/dL (<0.9 mmol/L) and/or triglyceride level >250 mg/dL (>2.8 mmol/L)
- Individuals with polycystic ovary syndrome
- Physical inactivity
- Other clinical conditions associated with insulin resistance (e.g., severe obesity, acanthosis nigricans, metabolic dysfunction–associated steatotic liver disease)
2. Individuals with pre-diabetes should be tested annually.
3. Individuals who had GDM should be tested every 1–3 years.
4. For all others, testing should begin after age 35.
5. If results are normal, testing should be repeated at an interval of 3 years.
6. Individuals in other high-risk groups — people with HIV, exposure to high-risk medicines, history of pancreatitis — should be monitored closely
- Individuals having acute pancreatitis should be screened for 3 -6 months after an episode and annually thereafter
- Individuals with cystic fibrosis should be tested annually from the age of 10
- Post transplantation after the individual is stable
Monogenic diabetes syndrome
<5% of individuals harbor monogenic defects of β-cell dysfunction in neonates causing neonatal diabetes and maturity-onset diabetes of the young (MODY).
- Neonates diagnosed with diabetes in the first 6 months of life should have genetic testing for neonatal diabetes and
- Children and young adults with diabetes who do not have typical characteristics of T1D or T2D and family history of diabetes in successive generations should have genetic testing for MODY.
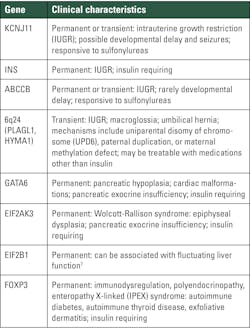
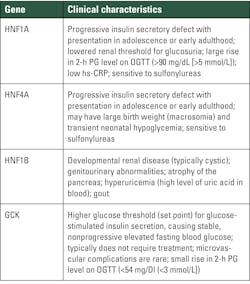
Genes causing monogenic diabetes syndrome are described in Tables 3 and 4.6
Gestational diabetes mellitus
Gestational diabetes mellitus (GDM) is a metabolic disorder characterized by increased blood sugar levels during the second and third trimester of the pregnancy in some women. It is associated with pancreatic β-cell dysfunction or delayed response to glucose levels and substantial insulin resistance due to release of placental hormones (human placental lactogen, estrogen, and progesterone).8 GDM poses risks for the mother, fetus, and neonate.2
Screening and diagnosis for GDM can be performed using either of the following two approaches:
Use of artificial intelligence in diabetes care
Artificial intelligence (AI) has been transforming every field including the medical field. A new article published in the journal Healthcare and Rehabilitation mentions how AI is transforming diabetes care.10 By analyzing data from blood sugar levels, medical history, and even retinal scans, AI tools can predict diabetes subtypes, identify high-risk patients, and tailor solutions to individual needs — with improved accuracy, reducing healthcare costs and addressing critical gaps in diagnosis, treatment, and daily management.
AI-enhanced continuous glucose monitoring systems not only merely report glucose trends but also anticipate hypoglycemic events hours in advance, offering patients critical time to intervene.10 Intelligent insulin delivery systems are now available to predict glucose monitoring and personalized treatment plans.10
Conclusion
Though diabetes is on the rise, artificial intelligence and remote monitoring systems have the capability to proactively monitor patients, provide personalized care, and save lives. However, care needs to be taken to ensure the data are safe and secure. Hence, healthcare professionals, IT professionals, and regulatory authorities must work together to ensure that patients benefit from newer technologies and at the same time remain safe and secure.
References
- Diabetes Atlas 11th Edition 2025. IDF. Published 2025. Accessed May 28, 2025. https://diabetesatlas.org/media/uploads/sites/3/2025/04/IDF_Atlas_11th_Edition_2025.pdf.
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: Standards of care in diabetes-2025. Diabetes Care. 2025;48(Supplement_1):S27-S49. doi:10.2337/dc25-S002.
- Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021;64(12):2609-2652. doi:10.1007/s00125-021-05568-3.
- Selvin E. Hemoglobin A1c-using epidemiology to guide medical practice: Kelly west award lecture 2020. Diabetes Care. 2021;44(10):2197-2204. doi:10.2337/dci21-0035.
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, Natural History, and Prognosis. Diabetes. 2017;66(2):241-255. doi:10.2337/db16-0806.
- Carmody D, Støy J, Greeley SAW, Bell GI, Philipson LH. A clinical guide to monogenic diabetes. In: Genetic Diagnosis of Endocrine Disorders. Elsevier; 2016:21-30.
- De Franco E, Flanagan SE, Houghton JAL, et al. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015;386(9997):957-963. doi:10.1016/S0140-6736(15)60098-8.
- Mittal R, Prasad K, Lemos JRN, Arevalo G, Hirani K. Unveiling gestational diabetes: An overview of pathophysiology and management. Int J Mol Sci. 2025;26(5):2320. doi:10.3390/ijms26052320.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768-773. doi:10.1016/0002-9378(82)90349-0.
- Ma S, Zhang M, Sun W, et al. Artificial intelligence and medical-engineering integration in diabetes management: Advances, opportunities, and challenges. Healthcare and Rehabilitation. 2025;1(1):100006. doi:10.1016/j.hcr.2024.100006.
To take the test online go HERE. For more information, visit the Continuing Education tab.
About the Author

Rajasri Chandra, MS, MBA
is a global marketing leader with expertise in managing upstream, downstream, strategic, tactical, traditional, and digital marketing in biotech, in vitro diagnostics, life sciences, and pharmaceutical industries. Raj is an orchestrator of go-to-market strategies driving complete product life cycle from ideation to commercialization.