Glycated albumin – utility and distinction vs A1C and fructosamine
In 2019, there were an estimated 463 billion people living with diabetes, and that number is predicted to rise to 578 million by 2030. Total diabetes-related health expenditures reached USD $760 billion in 2019, and the economic impact of diabetes is expected to continue growing.1 Therefore, prevention and treatment of diabetes are urgent issues throughout the world. However, since there are no subjective symptoms of hyperglycemia, regular examinations of the status of glycemic control using glycemic control markers, including hemoglobin A1c (A1C or HbA1c) and fructosamine,2 are important. Recently, clinicians in the United States have begun measuring a new glycemic control marker, glycated albumin, (GA), which offers its own utility as a specific, intermediate glycemic control marker.
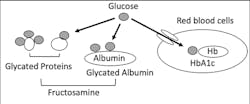
In blood, there are lots of proteins, including albumin, globulins and hemoglobin. These blood proteins will react with plasma glucose and then generate glycated proteins, including glycated albumin, glycated globulins and A1C (Figure 2). Levels of these glycated proteins reflect plasma glucose levels during the life span of each protein in the blood.3
Comparisons between A1C and GA
Since hemoglobin localizes in red blood cells (RBC), the A1C levels are in direct proportion to the glucose concentration in RBC and the life span of RBC. It’s known that glucose transport across the RBC membrane has inter-individual heterogeneity in glucose gradients across RBC membranes.4 In addition, A1C may not accurately reflect glycemic control in the presence of hemoglobinopathy, hemolytic anemia, or other conditions that affect RBC life span.5 The ADA Standards of Medical Care in Diabetes 2020 states that, when using A1C to diagnose diabetes, it is important to recognize that A1C is an indirect measure of average blood glucose levels and to take other factors into consideration that may impact hemoglobin glycation independently of glycemia. Such factors include hemodialysis, pregnancy, HIV treatment, age, race/ethnicity, pregnancy status, genetic background and anemia/hemoglobinopathies.2
GA, which reflects blood glucose concentrations over two to three weeks, is defined as albumin containing lysine residues bound to glucose (glycated lysine), and the results of GA are provided as a ratio of glycated albumin in albumin.6 Since GA localizes throughout the whole body, including the blood, interstitial fluid, lymph and cerebrospinal fluid, the amount of GA is in direct proportion to the blood glucose concentration and albumin metabolism. Because GA levels are not affected by the life span of RBC, it is a useful substitute for A1C when the interpretation of A1C is problematic (e.g., in the presence of hemoglobinopathies, iron deficiency and anemias).7 On the other hand, GA may not accurately reflect glycemic control in the presence of nephrosis, liver cirrhosis and other conditions that affect albumin metabolism.7
Since A1C is defined as the stable adducts of glucose to the N-terminal valine of the β-chain of hemoglobin, A1C has just one glycation site. On the other hand, GA is defined as albumin containing lysine residues bound to glucose (glycated lysine), and albumin has multiple glycation sites.9 Ueda et al. reported that the glycation speed was approximately 4.5 times faster for GA than A1C.10 Mo et al. reported that a 1 percent increase in A1C was associated with a 2.84 percent increase in GA.11
The glycation gap (GG) is a unique index that is calculated as the difference between intracellular RBC, A1C and the value predicted by regression on the extracellular fructosamine. GG provides useful information about A1C levels: People with higher-than-expected A1C levels from serum glucose levels are high glycators and those with lower-than-expected A1C are low glycators. Cohen RM et al. reported that high GG is a predictor of diabetic complications, including nephropathy and retinopathy,12 and GG might be explained by inter-individual heterogeneity in the erythrocyte transmembrane glucose gradient.4 Since GA is a major component of fructosamine, it could provide useful information, too. Examples include the results of the case cohort study of DCCT/EDIC13 and an Atherosclerosis Risk in Communities (ARIC) Study,14 in which A1C and GA were independently associated with diabetic complications.
Comparisons between GA and Fructosamine (GSP)
In blood, the amino groups of serum proteins bind to glucose to form stable ketoamines or fructosamines (1-amino-1-deoxyfructose). The nomenclature of fructosamine originated from its structure, and it is also called glycated serum protein (GSP).15 Fructosamine is an intermediate glycemic control marker that reflects blood glucose concentrations over three weeks. Since albumin is the largest component of the plasma proteins, representing more than 80 percent of the total molecules and 60 percent of the total plasma protein concentration, fructosamine has many common characteristics with GA. But the data has totally different properties due to its specificity and expressed units.
In addition, the serum sample should be used for the measurement because the protein concentration could vary between serum and plasma. Moreover, hemolyzed samples and samples obtained from patients who have received certain total parenteral nutrition (TPN) products are preferably avoided. Hemolysis samples may contaminate glycated proteins in the erythrocytes, and some stored TPN products may include glycated amino acids.16
The major differences between GA and fructosamine (GSP) as an intermediate glycemic control marker are:
1. Measuring substances and measurement unit
As mentioned above, fructosamine is measured as the fructosamine concentration in serum, and the unit is provided as µmol/L. Therefore, the data could be affected by the concentration, proportion, and half-life of each protein. In other words, fructosamine of the same glycemic control status could be different among individuals. Moreover, samples will be restricted to serum without hemolysis.
On the other hand, GA measures glycated albumin specifically, and provides the data as a ratio of glycated albumin in albumin. Therefore, the number of influencing factors of GA is reasonably less than that of fructosamine, while the major influencing factor of GA will be the presence of diseases that influence the half-life of albumin.7 GA is an evolved type of assay of fructosamine and GSP.
2. Standardization
Since fructosamine measurements include all the fructosamine groups on serum proteins, the setting of standard material would be difficult. Therefore, the standard method and material of fructosamine has not been provided yet. In contrast, the recommended reference-measurement procedure for GA determination has been published by the Japan Society of Clinical Chemistry, and the reference materials have also been distributed.
3. Outcomes
Peer-reviewed literature supports the use of GA as a good marker of glycemic control based on clinical outcomes for microvascular complications, including the DCCT/EDIC study,13 macrovascular complications, including the ARIC study,14 diabetes risk, prognosis in hemodialysis patients and predicting pregnancy outcomes. GA has been shown to be useful for intermediate term monitoring of glycemic control in patients with diabetes. In contrast, the literature for outcomes of fructosamine is limited.
Conclusion
In conclusion, A1C is the gold standard glycemic control marker, and GA is a useful substitute for A1C when interpretation of A1C is problematic and when monitoring the effects of changes in therapy in patients with diabetes or glycemic control status of gestational diabetes. GA could be a preferable marker than fructosamine (GSP) in the views of the affecter, including serum proteins other than albumin, concentration of proteins, standardization and outcomes.
References
- International Diabetes Federation. IDF Diabetes Atlas. Ninth edition. Brussels, Belgium: International Diabetes Federation. http://www.diabetesatlas.org. 2019.
- American Diabetes Association. Standards of Medical Care in Diabetes - 2020. Diabetes Care 2020 Jan; 43(Supplement 1): S14-S31.
- Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem 1987 33/12, 2153-2163.
- Khera PK, Joiner CH, Carruthers A, et al. Evidence for interindividual heterogeneity in the glucose gradient across the human red blood cell membrane and its relationship to hemoglobin glycation. Diabetes 2008; 57: 2445-52.
- Roy W. Beck, Crystal G. Connor, Deborah M. et al. The Fallacy of Average: How Using HbA1c Alone to Assess Glycemic Control Can Be Misleading. Diabetes Care 2017; 40: 994–999.
- Takei I, Hoshino T, Tominaga M, et al. Committee on Diabetes Mellitus Indices of the Japan Society of Clinical Chemistry – recommended reference measurement procedure and reference materials for glycated albumin determination. Ann Clin Biochem 2016; 53: 124-32.
- Shimizu I, Kohzuma T, and Koga M. A proposed glycemic control marker for the future: glycated albumin. J Lab Precis Med 2019; 4: 23.
- Basic performance of an enzymatic method for glycated albumin and reference range determination. Kohzuma T, Yamamoto T, Uematsu Y, Shihabi ZK, Freedman BI. J Diabetes Sci Technol 2011 Nov 1;5(6):1455-62.
- Iberg N, Flückiger R, Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem 1986; 261: 13542-5.
- Ueda Y, Matsumoto H. Recent topics in chemical and clinical research on glycated albumin. J Diabetes Sci Technol 2015; 9: 177-82.
- Mo Y, Ma X, Li H, et al. Relationship between glycated albumin and glycated hemoglobin according to glucose tolerance status: A multicenter study. Diabetes Res Clin Pract 2016; 115: 17-23.
- Cohen RM, LeCaire TJ, Lindsell CJ, et al. The relationship of prospective glycated hemoglobin to glycated serum proteins in incident diabetic retinopathy: implications of the glycation gap for mechanism of risk prediction. Diabetes Care 2008; 31: 151-3.
- Nathan DM, McGee P, Steffes MW, et al. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes 2014; 63: 282-90.
- Selvin E, Rawlings AM, Lutsey PL, et al. Fructosamine and Glycated Albumin and the Risk of Cardiovascular Outcomes and Death. Circulation 2015; 269-277.
- Armbruster DA. Fructosamine: Structure, Analysis, and Clinical Usefulness. Clin Chem 1987; 33/12: 2153-2163
- Tanabe H, Tsuji N, Endoh T, et al. Effect of total parenteral nutrition products on the measurement of glycated albumin by enzymatic method. Jpn J Clin Lab Automat 2003; 28: 134–8.
- Nathan DM, McGee P, Steffes MW, et al. Relationship of Glycated Albumin to Blood Glucose and HbA1c Values and to Retinopathy, Nephropathy and Cardiovascular Outcomes in the DCCT/EDIC Study. Diabetes 2014; 63(1): 282-290.
About the Author

Takuji Kohzuma, PhD
is Chief Researcher, Diagnostics and Clinical Development for Asahi Kasei Pharma Corporation, and is the inventor of the Lucica enzymatic method for glycated albumin (GA) measurement.