Automated in vitro test of albumin, creatine
Alere Afinion ACR is a fully automated in vitro diagnostic test for quantitative determination of albumin, creatinine, and albumin/creatinine ratio (ACR) in human urine. The measurements of these ratios aid in early diagnosis of nephropathy. The sample material is collected using the sampling device integrated in the test cartridge. Albumin is quantified using a solid phase immunochemical assay. The sample is automatically diluted and aspirated through a membrane coated with anti-albumin antibodies, which concentrates and immobilizes the albumin from the sample. A gold-antibody conjugate then binds to the immobilized albumin, resulting in a red-brown colored membrane. Excess gold-antibody conjugate is removed in a washing step. Creatinine is quantified using an enzymatic colorimetric test that involves four enzymatic steps. The test requires incubation with two distinct enzyme solutions. A colored end product is measured in one of the cartridge wells. The concentration of albumin, the concentration of creatinine, and the calculated ACR are displayed on the Alere Afinion AS100 Analyzer within six minutes. The AS100 Analyzer can also measure HbA1c in three minutes using the Alere’s CLIA-waived Afinion HbA1c assay. Alere
Assayed quality control material
AUDIT MicroControls Linearity LQ Special Diabetes is intended to simulate human patient serum samples for the purpose of determining linearity, calibration verification, and verification of reportable range. It is a ready-to-use liquid product, consisting of five levels that demonstrate a linear relationship to each other when assayed for C-peptide, fructosamine, and insulin. This product has an open vial stability of 7 days when stored at 2-8°C. It also provides Auditor QC, a free on-line, real time data reduction program that offers Levey-Jennings charts and peer group analysis for daily quality control product users, as well as linearity graphs and peer group analysis. Report results are given immediately in an “inspector friendly format” and stored on the Auditor QC website for future reference. AUDIT MicroControls
Hemoglobin A1c testing system
Bio-Rad’s D-100 Hemoglobin A1c testing system is a combination of high-performance liquid chromatography (HPLC) technology and innovative solutions to maximize workflow efficiency for laboratories that demand an accurate HbA1c result rapidly and effortlessly. The D-100 is fully automated and designed to meet the needs of medium- and high-volume clinical laboratories, for greater throughput, high quality results, and simplified workflow for A1c testing. The system delivers rapid results without sacrificing the ability to detect hemoglobin variants, which can interfere with interpretation of test results. BioRad
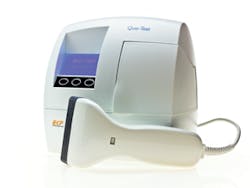
Automated hemoglobin A1c analyzer
EKF Diagnostics’ Quo-Test analyzer has been designed for easy and reliable HbA1c measurement for the monitoring and management of diabetes in point-of-care settings such as diabetes clinics and doctors’ offices. The Quo-Test HbA1c is a fully automated hemoglobin A1c analyzer that uses patented boronate fluorescence quenching technology to measure glycated hemoglobin from a 4 μl sample taken from a finger prick or venous whole blood. Sample results are available within four minutes and are reported in IFCC and DCCT standard units. Quo-Test is unaffected by most hemoglobin variants. EKF Diagnostics
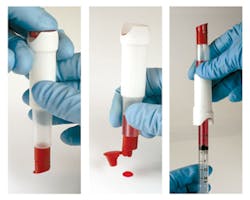
Rare blood control for glucose meters
Eurotrol’s CueSeeGlucose is a real blood control for validating precision and accuracy of glucose meters. Its real blood matrix makes it highly commutable and compatible with all glucose devices with minimal matrix effects. Low imprecision makes it comparable to human blood. The ACU-Drop II packaging form is a dual-chambered device keeping the fractions separated, preventing reactions between components of the desired matrix. Push a button to combine fractions, mix, and the sample is ready to use from the built-in dropper bottle or by attaching a syringe. Use for QC, calibration verification, proficiency testing, competency assessment, method validations, and lot comparisons. Eurotrol
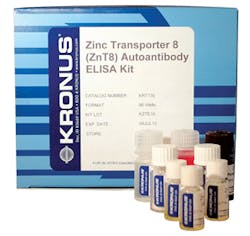
Zinc autoantibody ELISA assay kit
The KRONUS Zinc Transporter 8 Autoantibody (ZnT8Ab) ELISA Assay Kit is for the semi-quantitative determination of autoantibodies to zinc transporter 8 (ZnT8) in human serum, and may be useful as an aid in the diagnosis of type 1 diabetes mellitus (autoimmune mediated diabetes). Autoantibodies to ZnT8 (ZnT8Ab) have been detected in approximately 60 percent to 80 percent of patients at T1D onset and are more prevalent in children than in adults. Autoantibodies to ZnT8 have been found in T1D patients previously classified as autoantibody-negative, and when ZnT8Ab measurement is combined with the measurement of GADAb, IA-2Ab and IAA, the rate of detection of autoimmunity at diagnosis may increase. KRONUS
Automated adiponectin assay
Body Mass Index (BMI) has been used to measure health for years; however, debates surrounding its accuracy highlight that muscle, age, gender, or fitness are not accounted for. Patients with a “healthy” BMI could still be at risk of developing heart diseases, cancers, and type 2 diabetes mellitus (T2DM). Research suggests that abdominal visceral fat (AVF) has a stronger link to these chronic diseases, perhaps even predicting T2DM. The analyte adiponectin is inversely correlated with AVF, and allows a more accurate measurement of risk. The Randox automated adiponectin assay can be used on most biochemistry analyzers. Randox
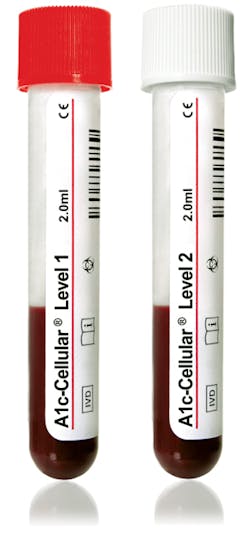
HbA1c control
Streck’s A1c-Cellular is an HbA1c control with intact red blood cells. It tests the entire HbA1c procedure, including the lysing of the red blood cells—a step omitted with other controls. This important step ensures the instrument, reagents, and technologist’s process are accurate. A1c-Cellular is ready-to-use; it reduces prep time with no reconstitution and no thawing required. It is appropriate for immunoassay and ionic exchange HPLC methodologies and assayed for popular chemistry instruments. It is available in plastic cap-pierceable vials which allow for autosampling by an analyzer. A1c-Cellular has 180-day closed-vial stability and 30-day open-vial stability. Streck
HPLC analyzer
The Tosoh G8 (Tosoh Automated Glycohemoglobin Analyzer HLC-723G8) is an FDA-cleared HPLC analyzer to aid in diagnosis of diabetes and identify patients who may be at risk for developing diabetes. HPLC methodology allows for the visual identification of hemoglobinopathies that can interfere with the HbA1c result. NGSP-Certified, the Tosoh G8 has demonstrated high pass rates and CVs of less than two percent in proficiency tests. Tosoh
About the Author
Sign up for our eNewsletters
Get the latest news and updates