Five clinical challenges laboratories need to understand about prostate cancer
Prostate cancer is common and costly. It is the fourth most prevalent of all cancer types globally and the most common in U.S. men.1,2 One in five men will receive a prostate cancer diagnosis and about 190,000 new cases will emerge each year in the U.S. alone, which equates to about 10 percent of all of the country’s cancer cases.2
Considered very rare 150 years ago, prostate cancer’s modern-day prevalence is attributed to, in part, advanced medical science and increased life expectancies.3 The latter is owing to prostate cancer’s increased occurrence in men aged 65 to 74 years old.2 The disease is also seen more commonly in African-American men, who are 1.8 times more likely to be diagnosed with the condition than Caucasian men and men of other ethnicities, and 2.2 times more likely to die from it.4
Prostate cancer is costly in terms of both the human and economic toll. It is the second leading cause of death for men in the U.S., where each year, more than 33,000 men die from the disease.5 That number increases more than tenfold for global mortality statistics.6 Although grave, on average, death rates have decreased, falling 2.1 percent year over year between 2008 and 2017.2 In 2000, there were 1.65 million men living with prostate cancer—a figure that grew to almost 3.2 million by 2017.2
Rising survivability rates have been linked to increased detection in the age of PSA screening, particularly in the early stages of the disease. This means that more men are living with cancer for years after their diagnoses. These men often fall into a treatment “gray zone,” and, for many, active surveillance is recommended in lieu of immediate treatment. The use of PSA testing also can lead to the detection of indolent cancers, however, and cause clinical dilemmas that place undue stress and anxiety on patients and clinicians.
As providers manage patients under the umbrella of value-based care, a number of factors come into play. These include clinical outcomes, patient satisfaction, resource allocation and cost-efficiency. In this environment, there is great focus on tools that can help support more targeted diagnostic testing and staging, the latter of which identifies disease progression and aggressiveness, and informs treatment decisions. These tests are designed to avoid over-testing, over-diagnosis and over-treatment.
To this end, test developers have focused attention on adjunct tests that can help identify at-risk patients more accurately, support appropriate staging and guide individualized treatment plans. Laboratories are pivotal in this effort. By understanding the challenges presented in prostate cancer care, laboratory personnel can add value to the diagnostic testing process and help influence clinically effective patient management.
Clinical laboratory challenges
1Prostate cancer is highly survivable, but notoriously heterogeneous
Survivability for prostate cancer is high—particularly when caught at earlier stages. The relative survival rate after five years is 98 percent.2 The disease is highly heterogeneous, however, with varied etiologies and diverse outcomes. Prostate cancer can be indolent, requiring no treatment, or it can be fast-growing and deadly. A number of tests inform diagnosis, pathological staging and treatment decisions, but gray areas and challenges exist.
Accurate pathological staging at the time of diagnosis affects survival and is key to developing an effective treatment protocol. Most cancers today are detected at the localized stage, and outcomes become less desirable with advanced disease.
Localized prostate cancer makes up 76 percent of the cancer diagnoses.2 With localized cancer, the disease is confined, and there is no indication that the cancer has spread outside the prostate. These cancers most often fall in the “gray zone,” a diagnostic range, characterized by tPSA levels between 4.0 and 10.0 ng/mL. This type of cancer has a 100 percent, five-year survival rate.2 Localized cancers can be concerning, however, if the pathological stage is high. The higher the stage, the more likely the cancer may progress to regional and distant cancer.
Regional prostate cancer—disease that has spread to the lymph nodes—also has a 100 percent five-year survival rate. These types of cancer make up 13 percent of cancer cases and require treatment.2 Distant prostate cancer—cancer that has metastasized—makes up 6 percent of diagnoses and has a 30.2 percent survival rate.2 Only 5 percent of cases are unstaged.2
2Screening is complicated and clinical gaps exist
Prostate cancer is a complicated disease that remains actively researched with great focus on diagnostic testing. Prostate cancer diagnostic strategies involve a combination of exams and tests that guide diagnostic decisions. There are limitations, however, and newer adjunct tests have emerged to add clinical value, specificity and clarity to those diagnostic strategies.
- Exams
Physical exams assess overall patient health and medical history, including the medical history of family members. This includes a digital rectal exam (DRE), a physical examination of the prostate, performed to identify abnormalities or lumps.
- MRI
More recently, multiparametric MRI has been looked at as a “novel promising tool” for detecting prostate cancer.7 While its use as a screening test is controversial, it might be helpful in mitigating over-diagnosis by enhancing identification of clinically significant cancer.
- PSA testing
Prostate-specific antigen (PSA) is a non-cancer-specific protein, produced by normal prostate gland cells, that plays a part in fertility. PSA exists in the blood in two forms—bound and unbound, the latter of which is identified as free PSA (fPSA). A total PSA (tPSA) measurement includes both the bound and unbound PSA. While increased PSA levels are associated with prostate cancer, they also are indicators of other biological conditions, such as benign prostate hyperplasia (BPH). Calculating the percentage of fPSA from tPSA (%fPSA) may not offer enough information for confident decision- making for biopsies, yet utilization of %fPSA has grown according to Medicare Claims data.
- Adjunct testing
Novel biomarkers and adjunct tests have emerged in recent years to address gray zones left by PSA testing and help stratify risk prior to biopsy. Tests differ in their approaches and vary in terms of functionality and operational integration. Ongoing research seeks to determine their clinical utility.
3 PSA testing remains controversial and presents clinical dilemmas
Since the 1980s, PSA testing has been the primary tool for guiding prostate cancer screening and diagnosis. Evidence is unclear as to its effect on patient mortality, but it has contributed to early detection. It also has led to over-diagnosis and over-treatment. In light of this, in 2012, the U.S. Preventive Services Task Force (USPTF) called for better testing and treatment options, and have since recommended individualizing PSA-based screening decisions based on evidence and frank discussions for men aged 55 to 69.8
The American Cancer Society (ACS) offered similar recommendations, stating that men with a 10-year life expectancy be given the opportunity to make an informed decision with their healthcare providers after receiving information about the associated “potential benefits, risks and uncertainties.”9 The ACS states that this conversation should begin at age 45 for African-American men.
Many of the “uncertainties” in PSA screening stem from a lack of specificity. Non-cancer factors can increase PSA levels, including advancing age, prostatitis, benign prostate hypereplasia (BPH), and some medications; likewise, PSA levels can decrease based on the use of certain herbs and medications.10 Specificity for PSA levels in the diagnostic gray zone (4 to 10 ng/mL) is low, and 75 percent of biopsies for men with tPSA levels in that range are negative.2 11
4 Confident biopsy, staging and treatment decisions require greater screening specificity
- Biopsies
Needle biopsies positively diagnose and appropriately stage prostate cancer. The aim of screening is to target the right patients for biopsies and avoid unnecessary procedures. Each year, 1.3 million men undergo biopsies, but more than 85 percent may not need them.12 This presents a clinical decision-making challenge.
- Staging
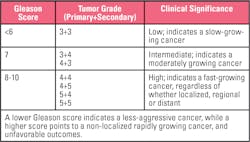
The Gleason system is the most-used grading framework for prostate cancer staging. The system uses the architectural features of prostate cancer cells, rating tissue according to distinctive patterns and histological grade group comparisons.
Pathologists add the two most prevalent ratings for categorized tissues (primary and secondary grade) to determine the Gleason score (generally omitting a tissue/tumor score of 1 to 2, as that is unlikely to indicate cancer).
The TNM (tumor, lymph node, metastases) system is used to classify disease at a population level and includes the Gleason score in the assessment. The goal is to have a staging system that draws from a growing body of molecular biology evidence from which a personalized treatment approach can emerge. Staging informs patient management protocols—from monitoring to treatment. Diagnostic specificity helps clinicians identify cancer behavior and patient risk to determine the appropriate care path.
5 Complementary prostate cancer tests are crucial for filling in diagnostic gaps
To fill the diagnostic gaps held by traditional prostate cancer screening and diagnostic tests, newer adjunct tests and novel biomarkers have been introduced into the landscape. These tests offer greater diagnostic clarity to guide pre-diagnosis biopsy decisions and post-diagnosis management decisions. Given the limitations of tPSA and %fPSA, for example, some adjunct tests combine measurements for different types of PSA, such as adding p2PSA, to create a prostate cancer probability score. These tests are designed to heighten specificity for patients with PSA screening results in the gray zone.
While adjunct testing can support better clinical decision-making for more targeted patient care, there are quality, efficiency and workflow factors for laboratories to consider. Some tests are FDA-approved, having undergone the testing requirements of the rigorous approval process. Other tests are laboratory-developed, filling a clinical need where a test may not exist. There may be cost and workflow advantages to tests that can be performed in-house and added to current analyzers or workflows versus those that are sent out. Adding diagnostic tests can increase costs, however, but these costs may be offset by a reduction in biopsies due to greater accuracy in selecting patients for biopsy. Researching current testing options in light of laboratory and organizational goals can ensure the best fit for clinical practice.
Conclusion
The goal of diagnostic testing is to give clinicians insights needed to make informed patient care decisions. For prostate cancer, gaps have existed, which have led to over-testing, over-diagnosis and over-treatment. As medical science continues to advance, giving way to tests that offer greater diagnostic clarity for care that is more individualized to patient needs, the laboratory can help bring value to patient management and its organizational and operational goals through adjunct testing.
References:
- World Health Organization (WHO). Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed April 14, 2020.
- National Cancer Institute (NCI). Cancer stat facts: prostate cancer. SEER. https://seer.cancer.gov/statfacts/html/prost.html. Accessed April 14, 2020.
- Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2(5):389–396.
- ZERO – The End of Prostate Cancer. African Americans and Prostate Cancer. https://zerocancer.org/learn/about-prostate-cancer/risks/african-americans-prostate-cancer. Accessed April 15, 2020.
- American Society of Clinical Oncology (ASCO). Cancer.net. Prostate cancer: statistics. https://www.cancer.net/cancer-types/prostate-cancer/statistics. Accessed April 15, 2020.
- Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68:394–424.
- Stabile A, Giganti F, Rosenkrantz AB et al. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nature Reviews Urology. https://www.nature.com/articles/s41585-019-0212-4, July 17, 2019. Accessed April 21, 2020.
- U.S. Preventive Service Task Force. Prostate cancer:screening.https://www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/prostate-cancer-screening recommendation statement. Accessed April 16, 2020.
- American Cancer Society. Cancer Facts & Figures 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed April 16, 2020.
- American Cancer Society. Screening tests for prostate cancer. https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/tests.html. Accessed April 20, 2020.
- Draisma G, Etzioni R, Tsodikov A. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–383.
- Alford AV, Brito JM, Yadav KK et al. The use of biomarkers in prostate cancer screening and treatment. Rev Urol. 2017;19(4):221–234.
- Altok M, Ward JF, Chapin B et. al. PD43-09 cost analysis of different prostate biopsy modalities. J Urol. 2017;197(4S):e821. https://www.researchgate.net/publication/315933153_PD43-09_COST_ANALYSIS_OF_DIFFERENT_PROSTATE_BIOPSY_MODALITIES. Accessed April 15, 2020.
- Amin MD et al. The eighth edition of AJCC Cancer Staging Manual: continuing to build a bridge from population-based to a more “personalized” approach to cancer staging. CA: Cancer J Clin. 2017;67:93–99.
- Prostate Conditions Education Council. Gleason score. https://www.prostateconditions.org/about-prostate-conditions/prostate-cancer/newly-diagnosed/gleason-score. Accessed April 20, 2020.
About the Author

Mark A. Reynolds, PhD
serves as Senior Director, Global Health Economics and Outcomes Research, at Beckman Coulter. Mark received his PhD degree in Pharmaceutical Chemistry from UCSF, and post-doctoral training in molecular biology from the University of Maryland. He has over 30 years of R&D leadership experience in the clinical diagnostics industry, with tenures at Biosite(Alere), Gen-Probe(Hologic), and Iris Intl. (Beckman Coulter) prior to joining Danaher. He currently leads Health Economic and Outcomes Research for Danaher’s Global Market Access Team.